b Y. B. Chavan College of Pharmacy, Rafiq Zakaria Campus, Aurangabad 431 001, India
In recent years, environmental protection laws and global warming effects have impelled the researchers to utilize renewable sources for chemical processes with minimum of waste or zero discharge. Therefore, the development of eco-friendly processes is one of the great challenges for organic chemists. On the other hand, heterocycles containing indazole moiety are of interest because they show some pharmacological and biological activities and often appears as an important structural component in compounds exhibiting high binding affinity for the estrogen receptor [1], inhibition of protein kinase C-b [2], 5-HT2, and 5-HT3 receptor antagonism [3], human immunodeficiency virus (HIV) protease inhibition [4], and antitumor activity [5]. In recent years, Phthalazine derivatives, constituting a bridgehead hydrazine are reported to possess a multiplicity of pharmacological properties including anticonvulsant [6], vasorelaxant [7], cardiotonic [8a], carbonic anhydrase inhibitors [8b], and activities. In addition, the titled compounds, 2H-indazolo[2, 1-b]phthalazinetrione compounds constitute a key structural motif in a number of natural and synthetic bioactive molecules along with unique electrical and optical properties. Therefore, synthesis of indazole group containgphthalazine derivatives using green protocols attracted significant attention.
In view of the importance of these heterocycles, various synthetic methods have been developed for the synthesis of 2H-indazolo[2, 1-b]phthalazine-trione. Recently, Esmat T. Kermani reported synthesis of 3, 4, 7, 8-tetrahydro-3, 3-dimethyl-11-aryl-2H-pyridazino[1, 2-a]indazole-1, 6, 9(11H)-triones and 2H-indazolo [2, 1-b]phthalazine-1, 6, 11(13H)-triones derivatives using acidic ionic liquidN, N-diethyl-N-sulfoethanammonium chloride ([Et3N-SO3H]Cl) under solvent free condition at 50 ℃ [9], Some other methods for synthesis of 1H-pyrazolo[1, 2-b]phthalazine-5, 10-diones has been also reported in the literature involving the use of acidic ionic liquid grinding method, [10], PEG-MDIL [11], wet cyanuric acid [12], SBA-Pr-NH2 [13], SO3H-functionalized mesoporous silica materials [14], supported polyphosphoric acid [15], dodecylphosphonic acid [16], Ce(SO4)2 [17], modified PEG-6000 [18], heteropolyacids [19], trimethylsilyl chloride [20], Mg(HSO4)2 [21], and cyanuric chloride [22] have been reported for the synthesis of this attractive family of compounds. These reported methodologies have shown good results in many instances. However, some of synthetic strategies also have limitations in terms of metal catalyst, expensive reagents, long reaction time, environmental hazard, harsh reaction conditions, tedious workup procedure, unsatisfactory yield, and use of homogeneous catalyst which are difficult in separation from reaction mixture and reuse. However, the development of green, mild and simpler procedures to eliminate the use and generation of hazardous substances is the foremost goal of green chemistry.
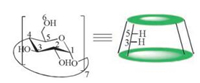
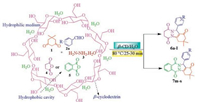
Cyclodextrins are cyclic oligosaccharides possessing hydrophobic cavity, which binds substrate selectively and catalyze chemical reactions with high selectivity. They catalyze reaction by supramolecular catalysis involving reversible formation of hostguest complexes by noncovalent bonding as seen in enzymes [23, 24]. β-Cyclodextrin is a cyclic heptamer composed of seven glucose units jointed head to tail by α-1, 4-links. The cavity size and the inner hydrophobicity are suitable for encapsulating a variety of guests such as aromatic compounds [25] (Fig. 1). It is widely accepted that the binding forces involved in the inclusion complex formation are Van der Waals interactions, hydrophobic interactions between guest molecules and β-CD [26]. Because of such behavior of β-CD is used as catalyst for varieties of organic reactions in aqueous medium [27]. To the best of our knowledge, the use of β-cyclodextrin for the synthesis of 2H-indazolo[2, 1-b] phthalazinetrione in water has not been reported.
|
Download:
|
| Fig. 1. Schematic of β-CD structure. | |
2. Result and discussion 2.1. Chemistry
In continuation of our work on β-CD and development of green methodologies [28], herein, we would like to report the synthesis of 2H-indazolo[2, 1-b]phthalazinetriones using β-cyclodextrin as a recyclable catalyst in water at 80 ℃ via one-pot four component reaction of dimedone(1), substituted aldehydes (2a), hydrazine hydrate (3) with succinic anhydride/pthalic anhydride (4) (Scheme 1).
|
Download:
|
| Scheme 1. General scheme for the synthesis of 2H-indazolo[2, 1-b]pthalazinetriones. | |
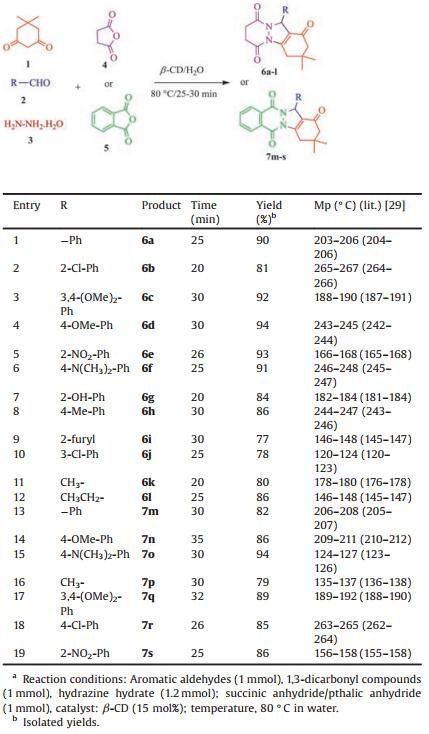
The catalytic role of β-CD for the synthesis of 2H-indazolo[2, 1-b]pthalazinetriones has been compared with various reported catalysts and found the present methodology developed green protocol for this transformation as well as the results found decent conversion of products within 25-30 min with good to excellent yields up to 80%-92% and results are summarized in (Table 1).
|
|
Table 1 Screening of reaction media for the synthesis of compound 6a. |
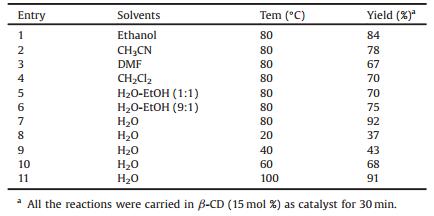
To optimize the reaction condition for the synthesis of 2H-indazolo[2, 1-b]pthalazinetrione, the reaction of dimedone (1), benzaldehyde (2a), hydrazine hydrate (3) with succinic anhydride (4) in the presence of β-CD in water at 80 ℃ as a standard model reaction. Without β-CD the reaction is sluggish to give lower yields (20%) with longer reaction times at higher temperature. The best result was obtained for 15 mol% of β-CD affording desired product without column chromatographic purification with 92% of yield within 25-30 min. The results indicate that a catalyst plays a critical role in this reaction. Encouraged by the initial success, we applied the optimal protocol to a variety of aldehydes. Generally, all the reactions were performed using 15 mol% of β-CD in water at 80 ℃ and all the reaction completed within 25-30 min to give the desired products in good to excellent yields.
Temperature also plays an important role, as surprisingly, at the low temperature there was a formation of trace amounts of product and required longer reaction time. As the temperature increases from room temperature to 100 ℃, the yields were found to increase while the reaction time decreases. We obtained the best results at 80 ℃ (Table 1, entry 7), hence all the reactions were performed at the temperature of 80 ℃.
The amount of catalyst was evaluated in the model reaction. The best result was obtained for 15 mol% of β-CD affording 92% of yield and using excess catalyst had no impact either on the rate or on the yield of product, whereas the yield was reduced by decreasing the amount of β-CD. A variety of other supramolecular catalyst, α-CD and γ-CD were also employed in the model reaction, and resulted in a low yield of product in a much longer reaction time. As β-CD is inexpensive and readily available, β-CD was selected as the catalyst to perform the reaction. The results have better performance than other reported catalysts including yields, reaction time and offers significant improvements in terms of simplicity and green aspects by avoiding high temperature.
Solvents also play an important role in the studied transformation.When the reaction was carried out under solvent free conditions, the targeted product was obtained with very low yields (35%) To find the best solvent for this transformation, model reaction was screened in H2O, MeOH, EtOH, CH3CN, CH2Cl2, DMF, H2O-EtOH (1:1), and H2O-EtOH (9:1) mixture. Among all these solvents, H2O was found to play an effective role in this transformation affording highest yields Therefore, H2O was selected as the solvent system for this transformation.
After optimizing the reaction conditions, we extended this process to other substrates. The scope of the reactions is illustrated with respect to various aldehydes and the results are summarized in Table 2. Both electron-rich and electron-deficient aromatic aldehydes, such as 4-methyl, 4-nitro, and 4-chlorobenzaldehydes reacted efficiently with dimedone, hydrazine hydrate with succinic anhydride to furnish the corresponding 2H-indazolo[2, 1-b]pthalazinetrione derivatives in good yields. The substituents present on the aromatic ring had shown some effect on the conversion. In all cases, the conversion was completed within 25-35 min with good to excellent yields of desired products without forming any by-products. The nature of functional group on the aromatic ring of aldehyde exerted a slight influence on the reaction time. The rate of reaction decreases in the case of arylaldehyde carrying an electron donating group in comparison to the unsubstituted. Similarly, hetero aromatic aldehydes like thiophene-2-carboxaldehyde reacted well to furnish the corresponding 2H-indazolo[2, 1-b]pthalazinetrione in good yield (Table 2, entry 9). The scope of the reaction was also investigated with aliphatic aldehydes as substrates which also reacted good to furnished the corresponding product (Table 2, entries 11, 12, 16). The compound was characterized by 1HNMR, 13CNMR, Mass.
|
|
Table 2 Synthesis of 3, 4, 7, 8-tetrahydro-3, 3-dimethyl-11-aryl-2H-pyridazino[1, 2-a]indazole 1, 6, 9(11H)-triones 6a-la and 2H-indazolo[2, 1-b]phthalazine-1, 6, 11(13H)-triones7m-s.a |
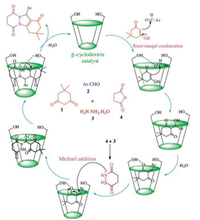
β-CD has a rigid conical molecular structure with a hydrophobic interior and a hydrophilic exterior. The internal hydrophobic cavity is the key structural feature of the β-CDs. It provides the ability to complex and holds a wide variety of inclusion molecules. To account for the very efficient catalysis by β-CD of this tandem reaction, wherein supramolecular catalyzed reactions are involved, it is proposed that β-CD with its seven free primaries -OH groups acting synergistically behaves as an efficient host and supramolecular catalyst (Scheme 2). Since the MCR is less efficient in the absence of β-CD, the catalyst appears to play an important role in increasing the efficiency of the reaction especially by activating aldehyde via coordination to the carbonyl group followed by the removal of 1, 3-cyclohexanedione and formation of a heterodiene in addition to aiding the water solubility of all the reactants. A subsequent Michael-type addition of phthalhydrazide, followed by cyclization was also facilitated by β-CD leading to the generation of the corresponding product in situ. In fact, β-CD not only activates carbonyl, amine, and enamine groups towards the nucleophilic attack of the hydroxyl group of β-CD, it can also converge and arrange the starting materials by the dual hydrogen-bonding properties. For these reasons, a small amount of the catalyst (15 mol%) was sufficient to promote the reaction efficiently.
|
Download:
|
| Scheme 2. Proposed reaction pathway for the synthesis of 2H-indazolo[2, 1-b]phthalazine-1, 6, 11(13H)-triones promoted by β-cyclodextrin. | |
2.2. Catalyst recyclability/reuse
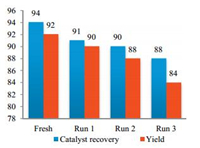
The catalyst recovery and reusability were studied by four cycles including the use of fresh catalyst for the synthesis of 6a (Table 2, entry 1). In every cycle, the catalyst was almost quantitatively recovered and after third and fourth use of catalyst decreasing yield is not much more significant which is shown in Fig. 2.
|
Download:
|
| Fig. 2. Reuse and recovery of β-CD and its effect on yield. | |
2.3. In vitro antibacterial activity
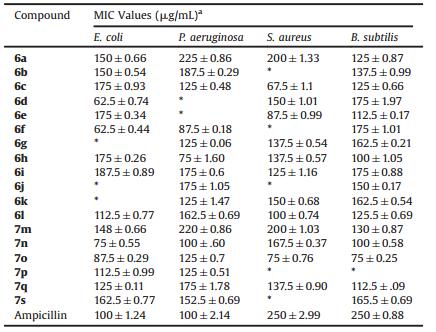
All the synthesized compounds were screened for in vitro antibacterial activity. The antibacterial activity was evaluated against four different bacterial strains such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis. Minimum inhibitory concentration (MIC) values were determined using g standard agar dilution method as per CLSI guidelines [30-33]. Ampicillin was used as a standard for the comparison of antibacterial activity. Dimethyl sulfoxide was used as solvent control. MIC values of the tested compounds were presented in (Table 3).
|
|
Table 3 Antimicrobial activity of 6a-l and 7m-s. |
Many of the synthesized compounds were found to show moderate to good antibacterial activity. From the antibacterial activity data (Table 3), scaffold containing 2H-indazolo[2, 1-b] pthalazinetrione shows considerable antibacterial activity. Also it was observed that compound 6c, 6d, 6e, 6f, 6l and 7o were the most active against most of the tested organisms. Unsubstituted phenyl analogue 6a and 7m shows lower activity against against E. coli and P. aeruginosa than activity against S. aureus and B. subtilis. Substituted phenyl analogues are more active than unsubstituted phenyl analogues against almost all of the tested organisms except for the compound 6b, and 6k where the activity has reduced due to substitution. Introduction of NO2 at ortho and -N-dimethyl group at para position of phenyl 6e, 6f and 7o increases the antibacterial activity against Escherichia coli, Pseudomonas aeruginosa of the tested organisms. Replacement of -OCH3 at 3 and 4 position of phenyl 6c & 7q increases the activity against S. aureus and B. subtilis. Introduction of -OH at 2 position of phenyl 6g and 7r shows moderate in activity against P. aeruginosa, S. aureus and B. subtilis compared to unsubstituted phenyl analogues. Introduction of -Cl at 3 position of phenyl 6b decrease the activity compared to unsubstituted phenyl analogues and also the activity is increase as compared to 4-Cl substituted phenyl analogues except against E. coli and S. aureus. Replacement of phenyl ring by other hetero rings like pyrrole ring 6i decrease the antibacterial activity against E. coli and P. aeruginosa than activity against S.aureus and B. subtilis. Also replacement of phenyl ring by aliphatic like formaldehyde shows moderate activity against S. aureusand B. subtilis than P. aeruginosa. Among these analogues 6c and 6d are most active compound against E. coli and P. aeruginosa and analogues 7o is most active against S. aureus and B. subtilis compared to standard. Thus, compound 6c, 6d and 7o are the most potent antibacterial compound from the present series.
The structure-activity relationship of the antibacterial activity evaluation against all bacterial strain were also explored. In the series of 2H-indazolo[2, 1-b]pthalazinetrione derivatives unsubstituted benzyl was moderately active against E. coli and B. subtilis (compounds 6a and 7m). Introduction of methyl group did not improve the activity. Replacement of hydroxyl group led to a slight increment the activity (compound 6g). Interestingly, compounds 6c, 6e, 6f, 7n and 7o with methoxy, nitro and N-dimethyl substituent's exhibited strong antibacterial activity against E. coli and Staphylococcus aureus with MIC values. Chemically, the nitro group possesses a unique combination of properties; it is strongly electron-withdrawing, small, polar, and can form hydrogen bonds. In addition, the nitro group can be bioactivated by enzymatic reduction to give reactive species. In many cases these reactive species are responsible for the biological effects of nitro drugs, in which case the nitroaromatic compound is acting as a pro-drug. The improvements of antibacterial activity were likely due to the presence of halogen, which was known to enhance lipophilicity and stability of the molecule. Changing the benzene ring to furan ring resulted also active analogues 6i against S. aureus and B. subtilis. From the structure-activity relationship study of these compounds, it was established that combination of 2H-indazolo [2, 1-b]pthalazinetrione with nitro, methoxy and N-dimethyl phenyl was favorable for the antibacterial activity. Unfortunately, the side aliphatic groups are not conducive to improve the antibacterial activity.
3. ConclusionIn summary, we have developed a one-pot, four-component ecofriendly protocol for the synthesis of 2H-indazolo[2, 1-b] pthalazinetrione derivatives catalyzed by supramolecular, biodegradable and reusable catalyst β-cyclodextrin. The salient features of the present methods are environmentally benign, mild neutral reaction conditions, good to excellent yields of products, simple and clean workup of the desired product without column chromatography and easy recovery as well as reuse of the catalyst. Among the synthesized compounds all compounds were tested for antibacterial activity. Based on the activity data, SAR for the series has been developed. From the series 6c, 6d, 6e, 6f, 6l and 7o serve as an important pharmacophore for the design and development of good lead as antibacterial agent indicating the future scope for optimization.
4. ExperimentalAll chemicals were purchased and used without any further purification. Melting points were determined on a open capillary tube and are uncorrected. 1H NMR spectra were recorded on a Varian As 400 MHz spectrometer in DMSO-d6; chemical shifts (d) are in ppm relative to TMS and coupling constant (J) are expressed in hertz (Hz). Mass spectra were taken on a Macro mass spectrometer (Waters) by electro-spray method. Progress of the reaction was monitored by thin layer chromatography on Merck's silica plates.
The synthesis of 6a: The mixture of dimedone (1 mmol), benzaldehyde (1 mmol), hydrazine hydrate (1.2 mmol) with succinic anhydride (1 mmol) were added in β-cyclodextrin (15 mol%) solution containing water, (10 mL). The resulting mixture was heated at 80 ℃. After completion of the reaction (monitored by TLC), the reaction mixture was poured into 20 mL water. Filtered the product and washed with hot water. The crude product was recrystallized with aqueous ethanol to afford the desired product. The filtered aqueous layer was cooled at 5 ℃ to recovery of β-CD by filtration. The recovered β-CD was reused for 2-3 consecutive runs in this reaction without any significant loss in yield and activity. The product was confirmed by melting point, Mass spectrum, 1H NMR, 13C NMR and elemental analysis.
The rest of substrates 6b-l and 7m-s were prepared by a procedure similar to that for 6a. The known compounds 6b-l and 7m-s showed satisfactory spectroscopic data in agreement with those reported in the literature.
AcknowledgmentsThis work was supported by Special Assistance Programme SAP, University Grants Commission, New Delhi, India, intended to encourage the pursuit of excellence and teamwork in advanced teaching and research to accelerate the realization of international standards in specific fields, and also Sophisticated Analytical Instrumentation Facility, Chandigarh, for providing characterization facility for this work.
| [1] | J.A. May, A.P. Dantanarayana, P.W. Zinke, M.A. McLaughlin, N.A. Sharif. 1-((S)-2-aminopropyl)-1H-indazol-6-ol: a potent peripherally acting 5-HT2 receptor agonist with ocular hypotensive activity. J. Med. Chem. 49 (2006) 318–328. DOI:10.1021/jm050663x |
| [2] | W. Han, J.C. Pelletier, C.N. Hodge. Tricyclic ureas: a new class of HIV-1 protease inhibitors. Bioorg. Med. Chem. Lett. 8 (1998) 3615–3620. DOI:10.1016/S0960-894X(98)00659-3 |
| [3] | H.D.H. Showalter, M.M. Angelo, E. Berman, et al., Benzothiopyranoindazoles, a new class of chromophore modified anthracenedione anticancer agents. Synthesis and activity against murine leukemias. J. Med. Chem 31 (1998) 1527–1539. |
| [4] | H.R. Shaterian, A. Hosseinian, M. Ghashang. Reusable silica supported poly phosphoric acid catalyzed three component synthesis of 2H-indazolo[2, 1-b] phthalazine-trione derivatives. Arkivoc 2 (2009) 59–67. |
| [5] | M. Kidwai, A. Jahan, R. Chauhan, N. Mishra, K. Neeraj. Dodecylphosphonic acid (DPA): a highly efficient catalyst for the synthesis of 2H-indazolo[2, 1-b] phthalazine-triones under solvent-free conditions. Tetrahedron Lett. 53 (2012) 1728–1731. DOI:10.1016/j.tetlet.2012.01.095 |
| [6] |
(a) F. Al-Assar, K. N. Zelenin, E. E. Lesiovskaya, I. P. Bezhan, B. A. Chahchir, Synthesis and pharmacological activity of 1-hydroxy-, 1-amino-, and 1-hydrazino-substituted 2, 3-dihydro-H-pyrazolo[1, 2-a]pyridazine-5, 8-diones and 2, 3-dihydro-H-pyrazolo[1, 2-b]phthalazine-5, 10-diones, Pharm. Chem. J. 36(2002) 598-603; (b) R. P. Jain, J. C. Vederas, Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase, Bioorg. Med. Chem. Lett. 14(2004) 3655-3658; (c) R. W. Carling, K. W. Moore, L. J. Street, et al. , 3-Phenyl-6-(2-pyridyl) methyloxy-1, 2, 4-triazolo[3, 4-a]phthalazines and analogues: high-affinity γ-aminobutyric acid-A benzodiazepine receptor ligands with α2, α3, and α5-subtype binding selectivity over α1, J. Med. Chem. 47(2004) 1807-1822. |
| [7] |
(a) S. Grasso, G. De Sarro, A. De Sarro, et al. , Synthesis and anticonvulsant activity of novel and potent 6, 7-methylenedioxyphthalazin-1(2H)-ones, J. Med. Chem. 43(2000) 2851-2859; (b) L. Zhang, L. P. Guan, X. Y. Sun, et al. , Synthesis and anticonvulsant activity of 6-alkoxy-[1, 2, 4]triazolo[3, 4-a] phthalazines, Chem. Biol. Drug Des. 73(2009) 313-319. |
| [8] |
(a) Y. Nomoto, H. Obase, H. Takai, et al. , Studies on cardiotonic agents. Ⅲ. Synthesis of 1-[1-(6, 7-dimethoxy-4-quinazolinyl)-4-piperidinyl]-3-substituted 2-imidazolidinone and 2-imidazolidinethione derivatives, Chem. Pharm. Bull. 38(1990) 2179-2183; (b) N. Berber, M. Arslan, E. Yavuz, C. Bilen, N. Gencer, Synthesis and evaluation of new phthalazine urea and thiourea derivatives as carbonic anhydrase inhibitors, J. Chem. 2013(2013) 1-8. |
| [9] | B. Pouramiri, E.T. Kermani. One-pot, four-component synthesis of new 3, 4, 7, 8-tetrahydro-3, 3-dimethyl-11-aryl-2H-pyridazino[1, 2-a]indazole-1, 6, 9(11H)-triones and 2H-indazolo[2, 1-b]phthalazine-1, 6, 11(13H)-triones using an acidic ionic liquid N, N-diethyl-N-sulfoethanammonium chloride ([Et3N? SO3H]Cl) as a highly efficient and recyclable catalyst. Tetrahedron Lett. 57 (2016) 1006–1010. DOI:10.1016/j.tetlet.2016.01.074 |
| [10] | X.C. Jia, J. Li, Y. Ding, B. Zhang, N. Wang, Y. Wang. A simple and green protocol for 2H-indazolo[2, 1-b]phthalazine-trionesusing grinding method. J. Chem. 1 (2013) 1–5. |
| [11] | B.M. Godajdar, M. Etamad, S. Soliemani. Green synthesis and dye propertied of 2H-indazolo [2, 1-b] phthalazinetriones by PEG-MDIL. Oriental J. Chem. 31 (2015) 1231–1236. DOI:10.13005/ojc |
| [12] | X. Wang, W.W. Ma, L.Q. Wu, F.L. Yan. Synthesis of 2H-indazolo[21-b] phthalazine-1, 6, 11(13H)-trione derivatives using wet cyanuric chloride under solvent-free condition. J. Chin. Chem. Soc. 57 (2010) 1341–1345. DOI:10.1002/jccs.v57.6 |
| [13] | G.M. Ziarani, N.H. Mohtasham, N. Lashgari, A. Badiei. Efficient one-pot synthesis of 2H-indazolo[21-b]phthalazinetrione derivatives with aminofunctionalized nanoporous silica (SBA-Pr-NH2) as catalyst. Res. Chem. Intermed. 41 (2015) 7581–7591. DOI:10.1007/s11164-014-1845-9 |
| [14] | A.A. Amiri, S. Javanshir, Z. Dolatkhah, M.G. Dekamin. SO3H-functionalized mesoporous silica materials as solid acid catalyst for facile and solvent-free synthesis of 2H-indazolo[21-b]phthalazine-1, 6, 11-trione derivatives. New J. Chem. 39 (2015) 9665–9671. |
| [15] | L. Nagarapu, R. Bantu, H.B. Mereyala. TMSCl-mediated one-pot, threecomponent synthesis of 2H-indazolo[2, 1-b]phthalazine-triones. J. Heterocycl. Chem. 46 (2009) 728–731. DOI:10.1002/jhet.v46:4 |
| [16] | H.R. Shaterian, F. Khorami, A. Amirzadeh, R. Doostmohammadi, M. Ghashang. Preparation of heterocyclic containing phthalazine skeletons: 2H-indazolo[21-b]phthalazine-1, 6, 11(13H)-triones. J. Iran. Chem. Res. 2 (2009) 57–62. |
| [17] | P. A. Grieco (Ed. ), Organic Synthesis in Water, Blackie Academic and Professional, London, 1998. |
| [18] | R. Breslow. Determining the geometries of transition States by use of antihydrophobic additives in water. Acc. Chem. Res. 37 (2004) 471–478. DOI:10.1021/ar040001m |
| [19] | N. Azizi, F. Aryanasab, L. Torkiyan, A. Ziyaei, M.R. Saidi. One-pot synthesis of dithiocarbamates accelerated in water. J. Org. Chem. 71 (2006) 3634–3635. DOI:10.1021/jo060048g |
| [20] | S. Grasso, G. DeSarro, N. Micale, et al., Synthesis and anticonvulsant activity of novel and potent 6, 7-methylenedioxyphthalazin-1(2H)-ones. J. Med. Chem. 43 (2000) 2851–2859. DOI:10.1021/jm001002x |
| [21] | N. Watanabe, Y. Kabasawa, Y. Takase, et al., 4-Benzylamino-1-chloro-6-substituted phthalazines: synthesis and inhibitory activity toward phosphodiesterase 5. J. Med. Chem. 41 (1998) 3367–3372. DOI:10.1021/jm970815r |
| [22] | Y. Nomoto, H. Obase, H. Takai, et al., 7-dimethoxy-4-quinazolinyl)-4-piperidinyl]-3-substituted 2-imidazolidinone and 2-imidazolidinethione derivatives. Chem. Pharm. Bull. 38 (1990) 2467–2471. DOI:10.1248/cpb.38.2467 |
| [23] | R. Breslowong, S.D. Dong. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 98 (1998) 1997–2011. DOI:10.1021/cr970011j |
| [24] | J.M. Desper, R. Breslow. Catalysis of an intramolecular Aldolcondensation by imidazole-bearing cyclodextrins. J. Am. Chem. Soc. 116 (1994) 12081–12082. DOI:10.1021/ja00105a070 |
| [25] |
(a) M. L. Bender, M. Komiyama, Cyclodextrin Chemistry, Springer-Verlag, New York, 1978; (b) K. Takahashi, Organic reactions mediated by cyclodextrins, Chem. Rev. 98(1998) 2013-2033. |
| [26] | J. Szejtli. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98 (1998) 1743–1754. DOI:10.1021/cr970022c |
| [27] |
(a) P. Rai, M. Srivastava, S. Yadav, J. Singh, S. Jagdamba, β-cyclodextrin: a biomimetic catalyst used for the synthesis of 4H-chromene-3-carbonitrile and tetrahydro-1H-xanthen-1-one derivatives, Catal. Lett. 145(2015) 2020-2028; (b) A. Kumar, V. D. Tripathi, P. Kumar, β-cyclodextrincatalysed synthesis of tryptanthrin in water, Green Chem. 13(2011) 51-54; (c) A. R. D. Kumar Shukla, Green Chem. 17(2015) 848-851; (d) S. Kumar, N. Ahmed, β-cyclodextrin/IBX in water: highly facile biomimetic one pot deprotection of THP/MOM/Ac/Ts ethers and concomitant oxidative cleavage of chalcone epoxides and oxidative dehydrogenation of alcohols, Green Chem. 18(2016) 648-656; (e) I. A. Azath, P. Puthiaraj, K. Pitchumani, One-pot multicomponent solventfree synthesis of 2-amino-4 H-benzo [b] pyrans catalyzed by per-6-aminoβ-cyclodextrin, ACS Sustain. Chem. Eng. 1(2013) 174-179; (f) S. Sukumari, I. A. Azath, K. Pitchumani, β-cyclodextrin-mediated acetic acid catalyzed diastereoselective mannich reaction in water, Synlett 23(2012) 2328-2332; (g) K. Kanagaraj, K. Pitchumani, Per-6-amino-β-cyclodextrin as a chiral base catalyst promoting one-pot asymmetric synthesis of 2-aryl-2, 3-dihydro-4-quinolones, J. Org. Chem. 78(2013) 744-751; (h) B. S. Londhe, U. R. Pratap, J. R. Mali, R. A. Mane, Synthesis of 2-arylbenzothiazoles catalyzed by biomimetic catalyst, β-cyclodextrin, Bull. Korean Chem. Soc. 31(8) (2010) 2329-2332. |
| [28] |
(a) A. V. Chate, U. B. Rathod, J. S. Kshirsagar, et al. , Ultrasound assisted multicomponent reactions: a green method for the synthesis of Nsubstituted 1, 8-dioxo-decahydroacridines using β-cyclodextrin as a supramolecular reusable catalyst in water, Chin. J. Catal. 37(2016) 146-152; (b) A. V. Chate, A. G. Tathe, P. J. Nagtilak, S. M. Sangle, C. H. Gill, Potent approach to heterocyclic skeletal by sequential one-pot three-component reaction of 2-amino -1-phenylethanone hydrochloride, aldehyde, and mercaptoacetic acid for the synthesis of novel thiazolidinones, Chin. J. Catal. 37(2016) 1997-2002; (c) A. V. Chate, S. B. Sukale, R. S. Ugale, C. H. Gill, Baker's yeast: An efficient, green and reusable biocatalyst for the one-pot synthesis of biologically important Nsubstituted decahydroacridine-1, 8-dione derivatives, Synth. Commun. 5(2016) 409-420. |
| [29] |
(a) R. Tayebee, M. Jomei, B. Maleki, et al. , A new natural based ionic liquid 3-sulfonic acid 1-imidazolopyridinium hydrogen sulfate as an efficient catalyst for the preparation of 2H-indazolo[2, 1-b]phthalazine-1, 6, 11(13H)-triones, J. Mol. Liq. 206(2015) 119-128; (b) R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri, M. Ghavidel, One-pot synthesis of aliphatic and aromatic 2H-indazolo[2, 1-b] phthalazine-triones catalyzed by N-halosulfonamides under solvent-free conditions, Tetrahedron 67(2011) 1930-1937; (c) J. Khurana, D. Magoo, Efficient one-pot syntheses of 2H-indazolo [2, 1-b] phthalazine-triones by catalytic H2SO4 in water-ethanol or ionic liquid, Tetrahedron Lett. 50(2009) 7300-7303. |
| [30] | R. Cruickshank, J. P. Duguid, B. P. Marmion, R. H. A. Swain, Medical Microbiology, 12th ed. , The Practice of Medical Microbiology, vol. 2, Churchill Livingstone Edinburgh, 1975, pp. 273-283. |
| [31] | A.H. Collins. Microbiological Methods, 2nd ed., Butterworth: London, 1976 . |
| [32] | Z.K. Khan. In vitro and in vivo screening techniques for bioactivity screening and evaluation. Proc. Int. Workshop UNIDO-CDRI (1997) 210. |
| [33] |
(a) B. Duraiswamy, S. K. Mishra, V. Subhashini, S. A. Dhanraj, B. Suresh, Studies on the antimicrobial potential of Mahonialeschenaultii Takeda root and root bark, Indian J. Pharm. Sci. 68(2006) 389-391; (b) A. R. Saundane, K. Rudresh, N. D. Satynarayan, S. P. Hiremath, Pharmacological screening of 6H, 11H-indole[3, 2-c]isoquinolin-5-ones and their derivatives, Indian J. Pharm. Sci. 60(1989) 379-383; (c) K. L. Therese, R. Bhagylaxmi, H. N. Madhavan, P. Deepa, In vitro antifungal susceptibility testing by agar dilution method to determine the MIC of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates, Indian J. Med. Microbiol. 24(2006) 273-279. (c) K. L. Therese, R. Bhagylaxmi, H. N. Madhavan, P. Deepa, In vitro antifungal susceptibility testing by agar dilution method to determine the MIC of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates, Indian J. Med. Microbiol. 24(2006) 273-279. |
 2017, Vol. 28
2017, Vol. 28