Tobacco mosaic virus (TMV), a positive-sensesingle stranded RNA virus, infects a wide range of plants, especially tobacco, vegetable and other members of the Solanaceae family. The virus is globally ubiquitous and causes great crop loss [1]. Therefore, TMV is an uncontrollable form of "plant cancer". Ribavirin and Ningnanmycin are common treatments for TMV infections under field conditions. However, Ribavirin has poor inactivation activity, and Ningnanmycin unstable and expensive [2, 3]. Therefore, the development of a novel, simple, and high-efficiency antiviral agents is a significant challenge in pesticide science [4].
Chalcone is a natural product, that exists in licorice, saffron and other medicinal plants. Chalcones have a wide range of pharmacological activities [5], such as anti-Alzheimer's disease [6], antitumor [7], antibacterial [8], insecticidal [9], anti-inflammatory [10], anti-oxidation [11], antiplatelet [12], and antiviral activities [13-15]. In our previous work, we reported a variety of chalcone derivatives and evaluated for their antiviral activity [16-18]. The results indicated that these compounds have moderate to good antiviral activity against TMV.
1, 3-Dichloropropene is a water-soluble and volatile compound that is mainly used as a pre-planting fumigant and nematicide. It is widely used in the US and other countries [19]. Its analogue, 1, 1-dichloropropene, also displays similar activities [20-23]. Pyridalyl, a commercially available 3, 3-dichloro-2-propenyloxy-substituted compound, has excellent controlling effects on various lepidopterous and thysanopterous insects. It shows no cross-resistance with the existing insecticides, such as synthetic pyrethroids, organic phosphates, nicotinic insecticides and ryanodine insecticides [24]. 1, 1-Dichloropropene compounds have drawn scientific attention as an interesting foundation for generating new lead compounds. Recently Liu et al. [25] synthesized several dichloroallyloxy-phenol derivatives by introducing various substituted phenyl groups into the pyrazole ring. The resulting insecticidal compounds are active against P. xylostella at 6.25 μg/mL and are more potent than the reference pyridalyl. In 2015, Yang and coworkers synthesized a series of the novel 1, 1-dichloropropene derivatives bearing diverse heterocycles with insecticidal activity against bollworm and P. litura [26]. Though, the 1, 1-dichloropropene group is an important and applicable functional group for designing the insecticide, the corresponding derivatives that contain the 1, 1-dichloropropene moiety with antiviral activities have not been reported.
In order to evaluate antiviral activity of 1, 1-dichloropropene compounds, the novel chalcone derivatives that contain the 1, 1-dichloropropene moiety were designed and synthesized. The physical characteristics were determined by 1H NMR, 13C NMR, IR and elemental analysis, and their antiviral activities were evaluated. The bioassay results indicated that the target compounds exhibited moderate to good inactivation activity against TMV. Compounds 7g, 7h, 7m, 7n, 7w, and 7z showed higher antiviral activity against TMV with EC50 values of 52.9, 45.6, 52.2, 53.8, 50.6, and 53.4 μg/mL, respectively, compared with Ribavirin (145.1 μg/mL). Furthermore, the interactions between target compounds and tobacco mosaic virus coat protein (TMV CP) were investigated by the fluorescence spectroscopy and microscale thermophoresis (MST).
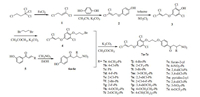
2. Results and discussion 2.1. ChemistryThe synthetic route of chalcone derivatives that contain the 1, 1-dichloropropene moiety is summarized in Scheme 1. Intermediate 1 can be easily prepared with 1, 1, 1, 3-tetrachloropropane [21]. Hydroquinone, 1, 1, 3-trichloroprop-1-ene 1 and K2CO3 were refluxed in CH3CN for 5 h to obtain intermediate 2 after solvent removal and dichloromethane extraction. Intermediate 2 and diethylamine as a catalyst were stirred in toluene. The mixture was slowly added to a solution of SO2Cl2. Then, the reaction mixture was stirred at 56 ℃ for 5 h to produce intermediate 3 [27]. Intermediate 3, K2CO3, KI, and 1, 3-dibromopropane were stirred at room temperature for 12 h to yield intermediate 4. Intermediates 5 and 6 were prepared in accordance with previously reported methods [25, 26]. Finally, intermediates 4 and 6 were reacted by etherification to yield the target compounds 7a-7z. The target products were characterized by 1H NMR, 13C NMR, IR, and elemental analysis. The data of spectroscopic characterization of the target compounds can be found in the Supporting Information.
|
Download:
|
| Scheme1. Synthesis of compounds 7a to 7z | |
2.2. Antiviral activity
The antiviral activities against TMV of the title compounds 7a-7z were determined via the half-leaf method [28, 29]. As shown in Table 1, the bioassay results indicated that all of the target compounds exhibited moderate to good activity against TMV at the concentration of 500 μg/mL. Among the targets, compounds 7g, 7h, 7m, 7n, 7w, and 7z showed better inactivation activity against TMV, with the inhibition rates of 93.1%, 94.8%, 93.5%, 92.9%, 94.0%, and 93.1%, respectively. These inhibition rates were similar to that of Ningnanmycin (94.1%) and superior to that of Ribavirin (72.9%). The other compounds displayed moderate inactivation activity against TMV.
|
|
Table 1 In vivo activity against TMV of the target compounds |
To establish the structure-activity relationships of the target compounds, the EC50 values of the target compounds' inactivation activities against TMV were evaluated. The evaluation results are presented in Table 2. All target compounds displayed excellent inactivation activity. Compounds 7g, 7h, 7m, 7n, 7w, and 7z showed antiviral activity against TMV with EC50 and EC90 values of 52.9, 45.6, 52.2, 53.8, 50.6, 53.4 and 332.9, 327.5, 331.5, 333.7, 331.0, 333.2 μg/mL, respectively. The inactivation activity of these compounds was better than that of Ribavirin (145.1 and 793.1 μg/mL). Among these six compounds, compound 7h exhibited the best inactivation activity against TMV and was similar to that of Ningnanmycin (46.9 and 329.4 μg/mL). Structureactivity relationship analysis revealed that differences in R groups have an important influence on inactivation activity. When the R groups were 4-OCH3-Ph(7h), 3-OCH3-Ph (7m), 4-OCH2CH3-Ph (7q), and 4-OCF3-Ph (7z), the corresponding compounds displayed good activity compared with 7e (2-Cl-Ph), 7f (2-Br-Ph), 7g (2-F-Ph) and 7k (2-CF3-Ph). Therefore, the introduction of electrondonating groups improves the anti-TMV activity of the chalcone compounds.
|
|
Table 2 In vivo EC50 values of compounds 7a–7z against TMV |
2.3. Interaction between 7h and TMV-CP
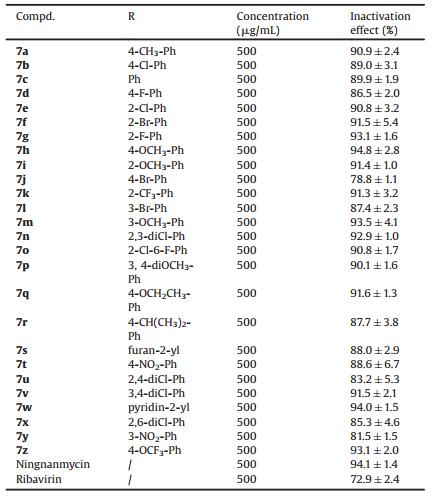
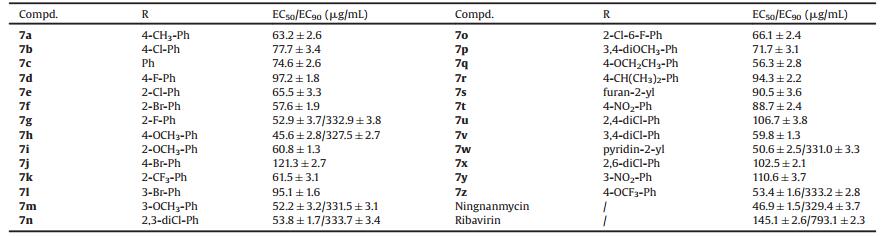
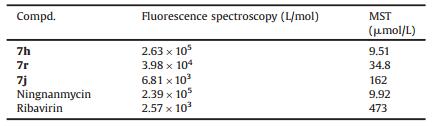
Fluorescence spectroscopy and MST analysis were used to investigate the interactions between target compounds and TMV CP [30]. The test methods are shown in the supporting data. The fluorescence spectrum showed that the binding constant Ka between 7h and TMV-CP was 2.63 ×105 L/mol, which was similar to that of Ningnanmycin (2.39 ×105 L/mol) and superior to that of Ribavirin (2.57 ×103 L/mol). Compound 7r (3.98 × 104 L/mol) showed moderate affinity and 7j (6.81 ×103 L/mol) showed weak affinity, as shown in Fig. 1 and Table 3. To further confirm the results of fluorescence spectroscopy, the Kd between compound 7h and TMV CP was investigated with MST. The result of MST analysis was consistent with the data presented by the fluorescence spectrum, as shown in Fig. 2 and Table 3. The Kd between 7h and TMV-CP was 9.51 μmol/L, which was higher than those of 7r (34.8 μmol/L) and 7j (162 μmol/L). Therefore, the result showed that compound 7h strongly interacted with TMV CP.

|
Download:
|
| Fig. 1. Fluorescence emission spectra of TMV-CP in the presence of 7h (A); Ningnanmycin (B); Ribavirin (C); 7r (D); and 7j (E). | |
|
|
Table 3 Binding constant of 7h, 7r, 7j, Ningnanmycin and Ribavirin |
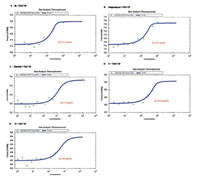
|
Download:
|
| Fig. 2. Microscale thermophoresis (MST) of 7h (A); Ningnanmycin (B); Ribavirin (C); 7r (D); and 7j (E). | |
3. Conclusion
In summary, we synthesized novel chalcone derivatives that contain a 1, 1-dichloropropene moiety. The anti-TMV activity of the target compounds 7a-7z was evaluated with the in vivo half-leaf method. Bioassay results showed that the target compounds were better than Ribavirin (145.1 μg/mL) against TMV. In particular, compound 7h possessed excellent inactivation activity against TMV and had an EC50 value of 45.6 μg/mL, which was similar to that of Ningnanmycin (46.9 μg/mL). In addition, fluorescence spectroscopy and MST showed that compound 7h strongly interacted with TMV CP. Further studies on the mechanism of inactivation are currently underway.
4. Experimental 4.1. SynthesisUnless otherwise stated, allreactants andreagents were purchased from commercial suppliers. The melting points were determined on an XT-4 binocular microscope (Beijing Tech Instrument, China) and were not corrected. Mass Spectra were obtained with a LC-MS 1100/MSD spectrometer (Agilent company, America). 1H NMR and 13C NMR (solvent CDCl3) spectral analyses were performed on a JEOL ECX 500 NMR spectrometer operated at 500 MHz for 1H NMR, 125 MHz for 13C NMR at room temperature and TMS as the internal standard. Elements were performed with an Elemental Vario-Ⅲ CHN analyzer, and IR spectra were acquired in KBr on a Bruker VECTOR 22 spectrometer. Analytical thin layer chromatography (TLC) was performed on silica gel GF254.
General procedure for the preparation of the key intermediate 6a-6z: Intermediates 6a-6z were prepared by performing previously reported synthetic procedures [24, 25]. A mixture of 4-hydroxychalcone (1.00 g, 4.2 mmol) and nitromethane (5.12 g, 83.9 mmol) in anhydrous ethanol was stirred. The mixture was refluxed for 2 h after the addition of KOH (0.28 g, 5.04 mmol). The reaction was monitored to completion via TLC. After the reaction system was cooled, its pH was adjusted with acid. Then, the mixture was filtered and the supernatant was discarded. The crude solid was purified via TLC on silica gel (petroleum ether/ethyl acetate = 3:1). The physical and chemical properties and spectroscopic characterization of the target compounds can be found in the Supporting information.
General synthetic procedures for the title compounds 7a-7z: A mixture of intermediates 4 (1.2 mmol), 6 (1.0 mmol) and potassium carbonate (2.0 mmol), with KI in acetone as the catalyst, was stirred and refluxed for 8 h. The solvent was evaporated in vacuo and the residue was isolated via column chromatography on silica gel (petroleum ether/dichloromathane = 1:1) to obtain the title compounds. The physical and chemical properties and spectroscopic characterization of the target compounds can be found in the Supporting information.
4.2. BioassayThe in vivo antiviral activities against TMV of the target compounds were examined. Ningnanmycin was used as the control. EC50 of the antiviral activity against TMV was determined. The assays were repeated thrice [28, 29].
4.3. Kd of compound 7h to TMV CPThe in vitro binding affinity of the title compounds on TMV-CP was performed by MST and fluorescence spectroscopy measurement as previously described [30].
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 21362004, 21562013) and Subsidy Project for Outstanding Key Laboratory of Guizhou Province in China (20154004).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.03.013.
| [1] | L. Bos. 100 years of virology: from vitalism via molecular biology to genetic engineering. Trends Microbiol. 8 (2000) 82–87. DOI:10.1016/S0966-842X(99)01678-9 |
| [2] | M.H. Chen, P. Li, D.Y. Hu, et al., Synthesis, antiviral activity, 3D-QSAR, and interaction mechanisms study of novel malonate derivatives containing quinazolin-4(3H)-one moiety. Bioorg. Med. Chem. Lett. 26 (2016) 168–173. DOI:10.1016/j.bmcl.2015.11.006 |
| [3] | S.X. Wang, Z. Fang, Z.J. Fan, et al., Synthesis of tetrazole containing 12, 3-thiadiazole derivatives via U-4CR and their anti-TMV activity. Chin. Chem. Lett. 24 (2013) 889–892. DOI:10.1016/j.cclet.2013.05.026 |
| [4] | H. Xiao, P. Li, D.Y. Hu, B.A. Song. Synthesis and anti-TMV activity of novel β-amino acid ester derivatives containing quinazoline and benzothiazole moieties. Bioorg. Med. Chem. Lett. 24 (2014) 3452–3454. DOI:10.1016/j.bmcl.2014.05.073 |
| [5] | Z.Y. Liang, X.S. Yang, Y. Wang, X.J. Hao, Q.Y. Sun. Two new chalcones from Fordia cauliflora. Chin. Chem. Lett. 21 (2010) 818–820. DOI:10.1016/j.cclet.2010.03.021 |
| [6] | S. Bag, S. Ghosh, R. Tulsan, et al., Design, synthesis and biological activity of multifunctional α, β-unsaturated carbonyl scaffolds for Alzheimer's disease. Bioorg. Med. Chem. Lett. 23 (2013) 2614–2618. DOI:10.1016/j.bmcl.2013.02.103 |
| [7] | H. Xu, X.M. Wang, X. Wei, J.Y. Li, K. Liu. A new chalcone from the aerial roots of Ficus microcarpa. Chin. Chem. Lett. 20 (2009) 576–578. DOI:10.1016/j.cclet.2009.01.013 |
| [8] | Y.H. Wang, H.H. Dong, F. Zhao, et al., The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 26 (2016) 3098–3102. DOI:10.1016/j.bmcl.2016.05.013 |
| [9] | T. Narender, Shweta, S. Gupta. A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro antileishmanial activity. Bioorg. Med. Chem. Lett. 14 (2004) 3913–3916. DOI:10.1016/j.bmcl.2004.05.071 |
| [10] | K. Damodar, J.K. Kim, J.G. Jun. Synthesis and pharmacological properties of naturally occurring prenylated and pyranochalcones as potent antiinflammatory agents. Chin. Chem. Lett. 27 (2016) 698–702. DOI:10.1016/j.cclet.2016.01.043 |
| [11] | F. Sonmez, S. Sevmezler, A. Atahan, et al., Evaluation of new chalcone derivatives as polyphenol oxidase inhibitors. Bioorg. Med. Chem. Lett. 21 (2011) 7479–7482. DOI:10.1016/j.bmcl.2011.09.130 |
| [12] | L.M. Zhao, H.S. Jin, L.P. Sun, H.R. Piao, Z.S. Quan. Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives. Bioorg. Med. Chem. Lett. 15 (2005) 5027–5029. DOI:10.1016/j.bmcl.2005.08.039 |
| [13] | J.H. Wu, X.H. Wang, Y.H. Yi, Y.H. Yi, K.H. Lee. Anti-AIDS agents 54. A potent antiHIV chalcone and flavonoids from genus Desmos. Bioorg. Med. Chem. Lett. 13 (2003) 1813–1815. DOI:10.1016/S0960-894X(03)00197-5 |
| [14] | H. Sharma, S. Patil, T.W. Sanchez, et al., Synthesis, biological evaluation and 3DQSAR studies of 3-keto salicylic acid chalcones and related amides as novel HIV-1 integrase inhibitors. Bioorg. Med. Chem. 19 (2011) 2030–2045. DOI:10.1016/j.bmc.2011.01.047 |
| [15] | G. Du, J.M. Han, W.S. Kong, et al., Chalcones from the flowers of Rosa rugosa and their anti-tobacco mosaic virus activities. Bull. Korean Chem. Soc. 34 (2013) 1263–1265. DOI:10.5012/bkcs.2013.34.4.1263 |
| [16] | D.D. Xie, Y. Xie, Y. Ding, J. Wu, D.Y. Hu. Synthesis of chiral chalcone derivatives catalyzed by the chiral cinchona alkaloid squaramide. Molecules 19 (2014) 19491–19500. DOI:10.3390/molecules191219491 |
| [17] | Z.H. Wan, D.Y. Hu, P. Li, D.D. Xie, X.H. Gan. Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules 20 (2015) 11861–11874. DOI:10.3390/molecules200711861 |
| [18] | B. A. Song, Z. W. Chen, D. Y. Hu, et al. Preparation of alpha-chalcone malonate derivative containing pyridine heterocycle and application for inhibition of cucumber mosaic virus and tobacco mosaic virus, CN104628631A. |
| [19] | N. Sakamoto, S. Saito, T. Hirose, et al., The discovery of pyridalyl: a novel insecticidal agent for controlling lepidopterous pests. Pest Manage. Sci. 60 (2004) 25–34. DOI:10.1002/(ISSN)1526-4998 |
| [20] | M.L. Feng, Y.F. Li, H.J. Zhu, et al., Design, synthesis, insecticidal activity and structure-activity relationship of 3, 3-dichloro-2-propenyloxy-containing phthalic acid diamide structures. Pest Manage. Sci. 68 (2012) 986–994. DOI:10.1002/ps.v68.7 |
| [21] | Z. Q. Wang, Y. L. Li, L. P. , Zhou, J. C. Wang, Pyridalyl insecticide, CN1860874A. |
| [22] | C. L. Liu, J. C. , Yang, J. F. Wang, et al. , Preparation of piperazine containing dihaloallyl ether compounds as pesticides, CN101921228A. |
| [23] | A.Y. Guan, Y.K. Qin, J.F. Wang, B. Li. Synthesis and insecticidal activity of novel dihalopropene derivatives containing benzoxazole moiety: a structure-activity relationship study. J. Fluor. Chem. 156 (2013) 120–123. DOI:10.1016/j.jfluchem.2013.09.003 |
| [24] | S. Saito, S. Isayama, N. Sakamoto, K. Umeda. Insecticidal activity of pyridalyl: acute and sub-acute symptoms in Spodoptera litura Larvae. J. Pestic. Sci. 29 (2004) 372–375. DOI:10.1584/jpestics.29.372 |
| [25] | M. Li, C.L. Liu, J. Zhang, et al., Design, synthesis and structure-activity relationship of novel insecticidal dichloro-allyloxy-phenol derivatives containing substituted pyrazol-3-ols. Pest Manage. Sci. 69 (2013) 635–641. DOI:10.1002/ps.2013.69.issue-5 |
| [26] | J. Li, Z.Y. Wang, Q.Y. Wu, G.F. Yang. Design, synthesis and insecticidal activity of novel 1, 1-dichloropropene derivatives. Pest Manage. Sci. 71 (2015) 694–700. DOI:10.1002/ps.2015.71.issue-5 |
| [27] | H. Sakaguchi, S. Matsuo, Regioselective chlorination method and secondaryamine catalysts for the production method of a 4-[(3, 3-dihalo-2-propenyloxy)-or (un)substituted benzyloxy]-2, 6-dichlorophenols, EP 1321449A1. |
| [28] | G.V. Jr. Gooding, T.T. Hebert. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 57 (1967) 1285. |
| [29] | J. Wu, Y.Y. Zhu, Y.H. Zhao, et al., Synthesis and antiviral activities of novel 14-pentadien-3-one derivatives bearing an emodin moiety. Chin. Chem. Lett. 27 (2016) 948–952. DOI:10.1016/j.cclet.2016.01.051 |
| [30] | M.H. Chen, D.Y. Hu, X.Y. Li, et al., Antiviral activity and interaction mechanisms study of novel glucopyranoside derivatives. Bioorg. Med. Chem. Lett. 25 (2015) 3840–3844. DOI:10.1016/j.bmcl.2015.07.068 |
 2017, Vol. 28
2017, Vol. 28