b Key Lab. of Biomass Energy and Material, Jiangsu Province, Nanjing 210042, China;
c National Engineering Lab. for Biomass Chemical Utilization, Nanjing 210042, China;
d Key and Open Lab. on Forest Chemical Engineering, State Forestry Administration, Nanjing 210042, China;
e Institute of Forestry New Technology, Chinese Academy of Forestry, Beijing 100091, China;
f 2011 Collaborative Innovation Center of Jiangxi Typical Trees Cultivation and Utilization in Jiangxi Agricultural University, Nanchang 330045, China
Weed reduces the quality and quantity of agricultural produce and causes huge economic loss to farmers [1, 2]. Since the middle of 1950s, synthetic herbicides have developed as a major tool to control weeds and significantly promoted the growth of the annual global food production [3-5]. Unfortunately, most of traditional herbicides were difficult to be degraded by microbes and easy accumulated in soil. The indiscriminate use of them resulted in various toxicological effects on the environment and living organisms [6-8]. Furthermore, extensive use of traditional herbicides always led to the herbicidal resistance of weeds. More and more evolving weed biotypes with herbicidal resistance have been reported in recent years [9, 10].
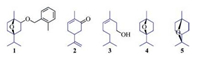
To overcome these problems, numerous efforts have been made to explore novel herbicides with little toxicity or a new mode of action [11-13]. Botanical herbicides attracted more and more attention due to their excellent biological activities and eco-friendly characteristics [13-15]. Monoterpene derivatives with a p-menthane (Fig. 1, 1-5) skeleton were one of the most important botanical pesticides [16-18]. Commercial herbicide cinmethylin (1) is an active pre-emergence herbicide against annual grass weeds and small-seeded broadleaf weeds [16]. p-Menthane type monoterpenes containing ketone, alcohol and other oxygen-containing functional groups (2-5) are efficient compounds in inhibiting the growth of green crops and the seed germination of weeds [17, 18].
|
Download:
|
| Fig. 1. Structure of p-menthane type botanical herbicides 1–5. | |
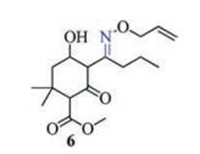
In recent years, Schiff base derivatives such as alloxydim 6 (Fig. 2) are used widely in the betterment of agricultural systems due to their wide spectrum of antifungal and herbicidal activities [19-22]. According to the principle of superposition of bioactive substructures, p-menthane type Schiff base derivatives should be a kind of compounds with high herbicidal activities [23]. In order to search new safety herbicides with high activity, a series of novel p-menthane type Schiff base derivatives 11a-11k were designed and synthesized (Scheme 1). The bioassay results showed that the target compounds displayed excellent herbicidal activities in pre-emergence treatment against annual ryegrass.
|
Download:
|
| Fig. 2. Structure of alloxydim (6). | |
|
Download:
|
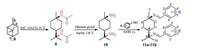
| Scheme1. Synthesis of Schiff base derivatives of cis-p-menthane-1, 8-diamines 11a–11k. | |
2. Results and discussion 2.1. Synthesis of N, N'-diacetyl-p-menthane-1, 8-diamines (9 and 9')
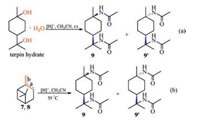
Cis- and trans-N, N'-diacetyl-p-menthane-1, 8-diamines (9 and 9') have been synthesized with a yield of 64% by Kovals'skaya and coworkers through the interaction of terpin hydrate with acetonitriles under the conditions of the Ritter reaction (Scheme 2, a) [24]. However, attempts to synthesize analogues of these compounds from terpenes and terpineols were unsuccessful in their work. In this investigation, we provided an efficient synthetic method for 9 and 9' using turpentine or pinene as starting material (Scheme 2b). Similar to the reaction of terpin hydrate with acetonitrile, the interaction between turpentine and acetonitrile also lead to the formation of isomeric mixtures in a ratio of 3.7:1. However, opposite to the work reported by Kovals'skaya and coworkers, these two isomers should be cis- and trans-N, N'-diacetyl-p-menthane-1, 8-diamines. The pure isomeric compounds were isolated from the isomer mixture of 9 and 9' by fractional recrystallization of the crude products in ethyl acetate.
|
Download:
|
| Scheme2. The synthesis of N, N'-diacetyl-p-menthane-1, 8-diamines (9 and 9') from terpin hydrate (a) or turpentine (b). | |
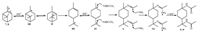
The reaction mechanism was speculated and illustrated in Scheme 3. Under the aqueous solution of H2SO4 60 wt. %, the double bond of α-pinene (7) or β-pinene (8) could be attacked by H+ and formed carbocation Ⅰ. Carbocation Ⅰ transformed to carbonium Ⅱ easily through a carbon-carbon bond cleavage reaction and then quickly rearranged to carbocation Ⅲ. The double bond of Ⅲ could be protonated and result in the formation of carbocation Ⅳ. Ⅳ could be trapped by acetonitrile and produce nitrilium ion intermediate Ⅴ, which subsequently reacted with water to the desired amide 9 and 9' through the formation of intermediates Ⅵ.
|
Download:
|
| Scheme3. The formation mechanism for 9 and 9' from turpentine. | |
2.2. Synthesis of cis-p-menthane-1, 8-diamines (10)
It has been reported that amide bonds could hydrolyzed to corresponding amines under the catalysis of acids, alkalis and metal salts [25, 26]. In order to explore an efficient synthetic method for cis-p-menthane-1, 8-diamines (10). The hydrolysis of 9 catalyzed by HCl, NaOH and FeCl3·6H2O in various reaction conditions were investigated. The results showed that almost no hydrolysis products could be observed in aqueous solutions of NaOH and FeCl3·6H2O. Under the treatment in aqueous solution of HCl, 9 could be hydrolyzed with high conversion. But most were mono hydrolysis by-products reacted in C8 position. The amino group in C8 position could be easily eliminated in acidic conditions. Thus, when the reaction was prolonged, the main products is N-acetyl-3-p-menthene-1-amine (Scheme 4, 13). Hydrolysis of 9 in NaOH solution of ethylene glycol at the reaction temperature higher than 170 ℃ led to the convenient formation of 10 with a yield of 84%. Further research showed that external temperature is an critical factor in this reaction. The hydrolysis rate of compound 9 decreased evidently with the decrease of reaction temperature and no products could be found at the reaction temperature lower than 140 ℃.

|
Download:
|
| Scheme4. Hydrolysis of compound 9 in aqueous solution of HCl. | |
2.3. Synthesis of cis-p-menthane type Schiff base derivatives (11a-11k)
In most cases, p-menthane-1, 8-diamine could react smoothly with various benzaldehyde derivatives. After stirring in ethanol for about 24 h at room temperature, over 95% of 10 could be transformed to Schiff base derivatives. Nevertheless, peripheral substituents in benzaldehyde could influence the reaction activity of them. Benzaldehyde derivatives substituted with electronwithdrawing groups could reduce the electron density of peripheral carbonyl groups and evidently accelerated this nucleophilic addition reaction (Table 1).
|
|
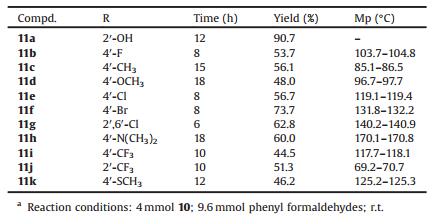
Table 1 The preparation of Schiff base derivatives of cis-p-menthane-1, 8-diamines 11a–11ka. |
2.4. Pre-emergence herbicidal effects evaluation of cis-p-menthane type Schiff base derivatives
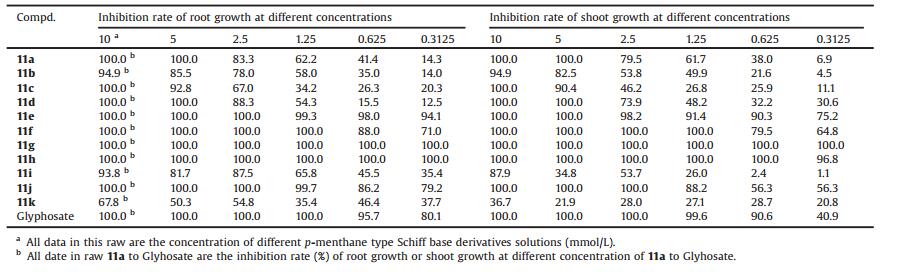
The pre-emergence herbicidal activities of 11a-11k against annual ryegrass were investigated. The toxicity regression equations and the IC50 and IC90 of 11a-11k were analyzed using DPS 9.5 software [27, 28]. The results in Table 2 indicated that some of p-menthane type Schiff base derivatives showed promising herbicidal activity against ryegrass. The root was inhibited completely at the concentration of 11f, 11g, 11h and 11j as low as 1.25, 0.31, 0.31 and 2.50 mmol/L, respectively. Meanwhile, the shoot was inhibited completely at the concentration of 1.25, 0.31, 0.63 and 2.50 mmol/L. The herbicidal activities of 11g-11h were even higher than Glyphosate, one of the most widely used commercial herbicides. The inhibitory rate of most p-menthane type Schiff base derivatives against root growth was lower than those of shoot growth, indicating that the test compounds had higher inhibition activity against the root growth than the shoot growth.
|
|
Table 2 Herbicidal activity of p-menthane type Schiff base derivatives 11a–11k against annual ryegrass. |
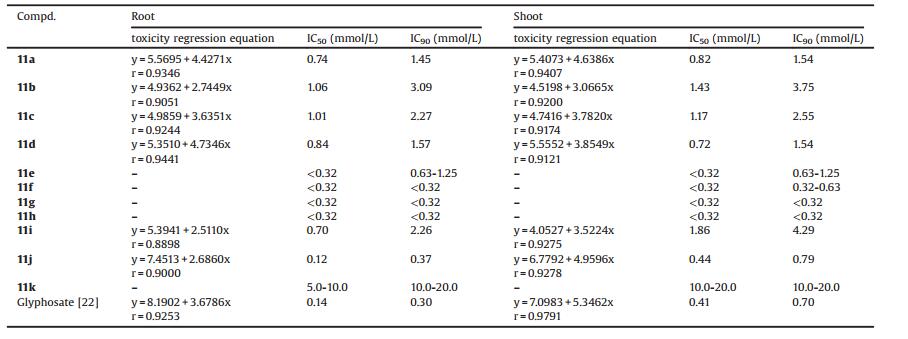
From the toxicity regression equations and the IC50 and IC90 analyzed in DPS 9.5 software, preliminary structure-activity relationship of 11a-11k could be obtained (Table 3). The presence of electron-withdrawing substituents on the phenyl ring is beneficial for herbicidal activity. p-Menthane type Schiff base derivatives with Cl and Br as phenyl substituents have much lower IC50 and IC90 than that substituted by CH3 and OCH3. With the increase of electron-withdrawing substituents, the activity would be greatly increased. The herbicidal activity of dichloro substituted derivatives 11g was much better than mono substituted chloro derivatives 11e. Substituents with herbicidal activity might also influence the activity of p-menthane type Schiff base derivatives. Organic amine groups were found to be beneficial to the activity of herbicides. Perhaps this is the most probable reason for the high herbicidal activity of 11h, which is substituted by two electrondonating N(CH3)2 groups in phenyl rings.
|
|
Table 3 Toxicity regression equations, IC50 and IC90 of p-menthane type Schiff base derivatives 11a–11j against annual ryegrass. |
3. Conclusion
Several novel cis-p-menthane type Schiff base botanical herbicides were synthesized and confirmed by FT-IR, 1H NMR, 13C NMR and ESI+-MS. The pre-emergence herbicidal activity evaluation showed most of these compounds had excellent herbicidal activities against annual ryegrass. Preliminary structure-activity relationship investigation showed that the herbicidal activities of them were mainly influenced by electronic property of substituent groups in phenyl ring.
4. Experimental 4.1. Synthesis of N, N'-diacetyl-p-menthane-1, 8-diamines17.0 g (0.125 mol) turpentine (containing 73% α-pinene and 20% β-pinene) or purity pinene (7 or 8) and 8.2 g acetonitrile (0.200 mol) were added in a 250 mL four-necked glass flask equipped with a mechanical agitator, a reflux condenser and a thermometer. 26.5 mL 60 wt. % aqueous solution of H2SO4 (0.300 mol) was added dropwise in 2 h under continuous stirring. The mixture was heated to 55 ℃ and then 4.1 g acetonitrile (0.100 mol) was added. The reaction temperature was increased to 75 ℃ stepwise in 2 h and reacted for 6 h. After reaction, the result mixture was transformed into 50 mL ice water and neutralized with 6 mol/L aqueous solution of NaOH. The viscous solid was separated and recrystallized in ethyl acetate to pure cis- and trans-N, N'-diacetyl-p-menthane-1, 8-diamines (9 and 9') as white solids.
4.2. Synthesis of cis-p-menthane-1, 8-diamines12.7 g 9 (0.05 mol), 10.0 g NaOH (0.25 mol) and 125 mL ethylene glycol were added in a 250 mL four-necked glass flask equipped with a magnetic agitator, a reflux condenser and a thermometer. The mixture was heated to reflux under continuous stirring and reacted for 13 h. After reaction, the mixture was cooled to room temperature and extracted with 150 mL CH2Cl2 for 4 times. The organic phase was collected and evaporated to afford crude products. The molar yield of 10 detected by GC under optimal reaction condition is 81.9%. After distillation at the vacuum of 2.00 kPa, cis-p-menthane-1, 8-diamines (10) with the purity of about 98% could be obtained as colourless liquid.
4.3. Synthesis of cis-p-menthane type Schiff base derivatives0.68 g 10 (4 mmol) was added in an 100 mL three-necked flask equipped with magnetic agitator, a thermometer and a reflux condenser. Under continuous stirring, 9.6 mmol phenyl formaldehyde derivatives dissolved in 20 mL dry ethanol was added dropwise. The resulted mixture was stirred at room temperature for 6-12 h. When the reaction was finished, the solvent was partially removed by vacuum evaporation and then corresponding pure Schiff base derivatives of cis-p-menthane-1, 8-diamines 11a-11k could be obtained.
4.4. Pre-emergence herbicidal effects evaluation of cis-p-menthane type Schiff base derivativesAnnual ryegrass was chosen to evaluate the pre-emergence herbicidal effects of 11a-11k under greenhouse conditions. In a typical procedure: Seeds of ryegrass were soaked in warm water 25 ℃ for 15 h before test. 20 mmol/L stock solutions of 11a-11k were prepared by dissolving 2 mmol 11a-11k with 0.25 ml DMF containing a drop of Tween-80 and diluted with double-distilled water in an 100 mL volumetric flask, respectively. Solutions of 11a-11k were diluted to 10 mmol/L, 5 mmol/L, 2.5 mmol/L, 1.25 mmol/L, 0.625 mmol/L and 0.3125 mmol/L, respectively.10 mL the solutions of 11a-11k with different concentrations were added to corresponding Petri dishes (9 cm diameter) lined with filter paper (9 cm diameter). Then each of the Petri dishes were added 10 seeds and cultivated in dark at 25 ℃. After cultivation for 5 days, the root and shoot growth inhibitory rates related to the control were determined. The experiments were conducted in three replicates with 10 seeds per plant for each concentration. The inhibition rates were calculated according to the equation listed below:
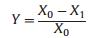
|
where Y, X0, X1 are the inhibition rates of the root or shoot growth, the length of root or shoot for blank control samples and the root or shoot length for test samples, respectively.
AcknowledgmentsThis work is funded by the Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (No. 2015BAD15B04), the National Natural Science Foundation of China (No. 31600466) and the Fundamental Research Funds for Jiangsu Key Laboratory of Biomass Energy and Material (No. JSBEM-S-201605).
| [1] | G.Y. Li, X.H. Qian, J.N. Cui, et al., Synthesis and herbicidal activity of novel 3-aminocarbonyl-2-oxazolidinethione derivatives containing a substituted pyridine ring. J. Agric. Food Chem. 54 (2006) 125–129. DOI:10.1021/jf051928j |
| [2] | J.Q. Huo, L.Y. Ma, Z. Zhang, et al., Synthesis and biological activity of novel N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amides derivatives. Chin. Chem. Lett. 27 (2016) 1547–1550. DOI:10.1016/j.cclet.2016.06.019 |
| [3] | L.Y. Zheng, R.M. Wu, W. Guo, et al., Design, synthesis and herbicidal activities of novel self-dispreading phenoxy carboxylic acid derivatives for the control of water hyacinth floating on the water surface. Chin. Chem. Lett. 26 (2015) 1008–1010. DOI:10.1016/j.cclet.2015.04.008 |
| [4] | M. Baalouch, A. De Mesmaeker, R. Beaudegnies. Efficient synthesis of bicyclo [3.2.1]octane-2, 4-diones and their incorporation into potent HPPD inhibitors. Tetrahedron Lett. 54 (2013) 557–561. DOI:10.1016/j.tetlet.2012.11.081 |
| [5] | Y. Xie, H.W. Chi, A.Y. Guan, et al., Synthesis and evaluation of substituted 3-(pyridin-2-yl) benzenesulfonamide derivatives as potent herbicidal agents. Bioorg. Med. Chem. 24 (2016) 428–434. DOI:10.1016/j.bmc.2015.08.037 |
| [6] | T. Hanazato. Pesticide effects on freshwater zooplankton: an ecological perspective. Environ. Pollut. 112 (2001) 1–10. DOI:10.1016/S0269-7491(00)00110-X |
| [7] | H.P. Singh, D.R. Batish, N. Setia, R.K. Kohli. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 146 (2005) 89–94. DOI:10.1111/aab.2005.146.issue-1 |
| [8] | D. Hernández-Moreno, A.E.M.F. Soffers, Wiratno, et al., Consumer and farmer safety evaluation of application of botanical pesticides in black pepper crop protection. Food Chem. Toxicol. 56 (2013) 483–490. DOI:10.1016/j.fct.2013.01.033 |
| [9] | X.L. Yue, H. Li, S.S. Liu, et al., N-Fluorinated phenyl-N'-pyrimidyl urea derivatives: Synthesis, biological evaluation and 3D-QSAR study. Chin. Chem. Lett. 25 (2014) 1069–1072. DOI:10.1016/j.cclet.2014.03.046 |
| [10] | X.H. Liu, Z.W. Zhai, X.Y. Xu, et al., Facile and efficient synthesis and herbicidal activity determination of novel 1, 2, 4-triazolo[4, 3-α]pyridin-3(2H)-one derivatives via microwave irradiation. Bioorg. Med. Chem. Lett. 25 (2015) 5524–5528. DOI:10.1016/j.bmcl.2015.10.064 |
| [11] | S.O. Duke, J.G. Romagni, F.E. Dayan. Natural products as sources for new mechanisms of herbicidal action. Crop Protect. 19 (2000) 583–589. DOI:10.1016/S0261-2194(00)00076-4 |
| [12] | B.L. Wang, Y.H. Li, J.G. Wang, Y. Ma, Z.M. Li. Molecular design, synthesis and biological activities of amidines as new ketol-acid reductoisomerase inhibitors. Chin. Chem. Lett. 19 (2008) 651–654. DOI:10.1016/j.cclet.2008.04.009 |
| [13] | Y. Huang, L. Li, J. Liu, L. Wei. Botanical pesticides as potential rotifer-control agents in microalgal mass culture. Algal Res. 4 (2014) 62–69. DOI:10.1016/j.algal.2013.08.001 |
| [14] | Y.Q. Gao, L.L. Li, H. Chen, et al., High value-added application of rosin as a potential renewable source for the synthesis of acrylopimaric acid-based botanical herbicides. Ind. Crops Prod. 78 (2015) 131–140. DOI:10.1016/j.indcrop.2015.10.032 |
| [15] | M. Zhang, X.H. Xu, Y. Cui, L.G. Xie, C.H. Kong. Synthesis and herbicidal potential of substituted aurones. Pest Manag. Sci. 68 (2012) 1512–1522. DOI:10.1002/ps.v68.11 |
| [16] | B.T. Grayson, K.S. Williams, P.A. Freehauf, R.R. Pease, W.T. Ziesel, R.L. Sereno, R.E. Reinsfelder. The physical and chemical properties of the herbicide cinmethylin (SD 95481). Pest Manag. Sci. 21 (1987) 143–153. DOI:10.1002/ps.v21:2 |
| [17] | S.F. Vaughn, G.F. Spencer. Synthesis and herbicidal activity of modified monoterpenes structurally similar to cinmethylin. Weed Sci. 44 (1996) 7–11. |
| [18] | J. Li, J.J. Li, Y.Q. Gao, et al., Taking advantage of a sustainable forest resource in agriculture: A value-added application of volatile turpentine analogues as botanical pesticides based on amphipathic modification and QSAR study. ACS Sustain. Chem. Eng. 4 (2016) 4685–4691. DOI:10.1021/acssuschemeng.6b00819 |
| [19] | S. Samadhiya, A. Havle. Synthetic utility of Schiff bases as potential herbicidal agents. Orient. J. Chem. 17 (2001) 119–122. |
| [20] | N. Aggarwal, R. Kumar, P. Dureja, D.S. Rawat. Schiff bases as potential fungicides and nitrification inhibitors. J. Agric. Food Chem. 57 (2009) 8520–8525. DOI:10.1021/jf902035w |
| [21] | A. Sandín-España, B. Sevilla-Morán, L. Calvo, M. Mateo-Miranda, J.L. AlonsoPrados. Photochemical behavior of alloxydim herbicide in environmental waters. Structural elucidation and toxicity of degradation products. Microchem. J. 106 (2013) 212–219. DOI:10.1016/j.microc.2012.07.003 |
| [22] | S.J. Zhu, S.C. Xu, J. Wang, Z.D. Zhao, J.X. Jiang. Synthesis and herbicidal activities of p-menth-3-en-1-amine and its schiff base derivatives. J. Agric. Food Chem. 64 (2016) 9702–9707. DOI:10.1021/acs.jafc.6b03977 |
| [23] | Y.C. Guo, J. Li, J.L. Ma, et al., Synthesis and antitumor activity of a-aminophosphonate derivatives containing thieno[2, 3-d]pyrimidines. Chin. Chem. Lett. 26 (2015) 755–758. DOI:10.1016/j.cclet.2015.03.026 |
| [24] | S.S. Kovals'skaya, N.G. Kozlov, T.S. Tikhonova. Stereoselective synthesis of N, N'-diacyl-p-menthane-1, 8-diamines. Chem. Nat. Compd. 25 (1989) 552–557. DOI:10.1007/BF00598074 |
| [25] | Y. Shimizu, H. Morimoto, M. Zhang, T. Ohshima. Microwave-assisted deacylation of unactivated amides using ammonium-salt-accelerated transamidation. Angew. Chem. Int. Ed. 51 (2012) 8564–8567. DOI:10.1002/anie.201202354 |
| [26] | P.R. Sultane, T.B. Mete, R.G. Bhat. Chemoselective N-deacetylation under mild conditions. Org. Biomol. Chem. 12 (2014) 261–264. DOI:10.1039/C3OB41971A |
| [27] | Q.Y. Tang, M.G. Feng. DPS Data Processing System for Practical Statistics, Beijing: Science Press, 2002 . |
| [28] | M.Z. Huang, K.L. Huang, Y.G. Ren, et al., Synthesis and herbicidal activity of 2-(7-fluoro-3-oxo-3, 4-dihydro-2H-benzo[b][1, 4] oxazin-6-yl)isoindoline-1, 3-diones. J. Agric. Food Chem. 53 (2005) 7908–7914. DOI:10.1021/jf051494s |
 2017, Vol. 28
2017, Vol. 28