b Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
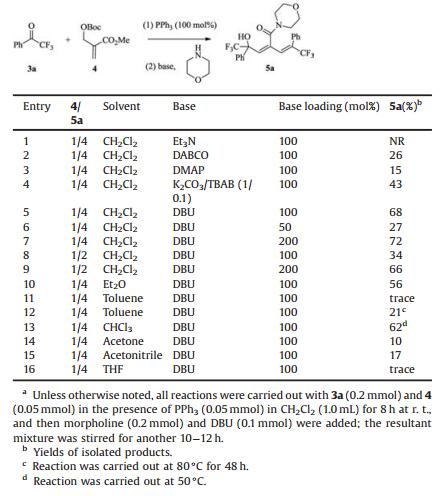
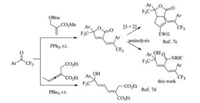
Dienamides are a structural motif prevalent in numerous biologically related molecules and are also key reactive intermediates in the synthesis of some small-molecule natural products[1-3]. For instances, hexadienamide 1 was reported to undergo a pivotal Pd-catalyzed bis-cyclization in the stereocontrolled synthesis of indole alkaloid spirotryprostatin B[4]. Dienamide 2, detected in valproic acid (VPA)-treated rats, is a glycine-conjugated metabolite of VPA[5]. Butadiene-imide derivative T-2639, showed inhibitory potency on the production of plasminogen activator inhibitor-1, is a promising antithrombotic drug candidate (Fig. 1)[6]. On the other hand, the CF3-substituted quaternary carbon center, particularly CF3-substituted tertiary alcohol, are common subunits in some bioactive molecules with appealing pharmacological activity[7], and their preparation methods have gained increasing interest among the synthetic community[8]. Though sorts of approaches for assembling dienamides have been documented[9-14], including metalcatalyzed diene-coupling reactions and Wittig olefination with α, β-unsaturated aldehydes, new methods enabling the expedient synthesis of CF3-bearing dienamides remain highly desirable and would prove valuable for screening bioactive dienamide-containing molecules as potential pharmceuticals.
|
Download:
|
| Fig. 1. Examples of dienamide-containing biologically interesting compounds. | |
In 2014, we developed a phosphine-promoted tandem vinylogous addition/lactonization/Wittig reaction between MBH carbonates and aryl trifluoromethyl ketones, and a series of trifluoromethylated vinyl butenolide products were obtained with controllable chemoselectivity (Scheme 1)[15, 16]. In this context, the in situ generated butenolides act as electrophilic alkene in a traditional phosphine-catalyzed [3 + 2] cycloaddition with MBH carbonate or allenoate (vide infra) to produce bicyclic lactones. In the continuing work, we found that treatment of the resulting reaction mixture with secondary alkylamine and an amidine-type base led to direct amidation of the lactone smoothly[17], giving trifluoromethylated dienamides with a tertiary alcohol moiety in a one-pot operation. Herein, we unveiled the outcome of this investigation.
|
Download:
|
| Scheme1. Phosphine-mediated reactions of trifluoromethyl ketones with MBH carbonates or bis-substituted allenoates. | |
2. Results and discussion
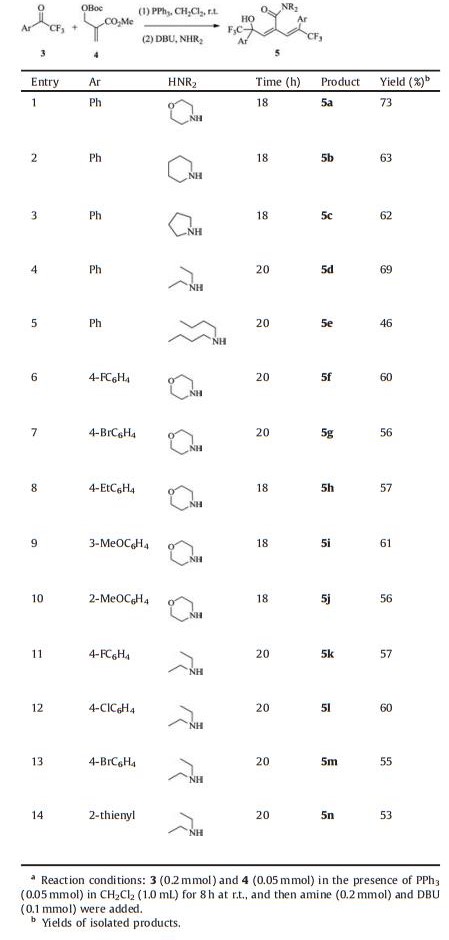
The reaction was first conducted with phenyl trifluoromethyl ketone 3a, allylic carbonate 4 and PPh3 in CH2Cl2 at r.t. for 8 h to produce butenolide compound as previously described [16c]. Then the in situ formed butenolide and cyclic secondary amine morpholine were reacted in the presence of a stoichiometric amount of 1, 8-diazabicyclo[5.4.0]undec-7-ene (DBU), the direct aminolysis product 5a was obtained uneventfully with satisfying chemical yield. A selection of bases, including Et3N, DABCO, 4-dimethylaminopyridine and K2CO3/tetrabutylammonium bromide, was evaluated and bicyclic amidine base DBU offered the best reaction efficiency (Table 1, entries 1–5). Either the use of amine and ketone in a 4:1 stoichiometry or raising the loading of DBU to 2 equiv. produced 5a in slightly improved yield, while decreasing the amount of morpholine or DBU employed markedly reduced the process efficiency (Table 1, entries 6–9). Solvent screening revealed that reactions worked in Et2O or CHCl3 as well, and CH2Cl2 was identified as medium of choice (Table 1, entries 10–16). Typically, with a ketone/carbonate ratio of 4:1 in the presence of 100 mol% of PPh3 in CH2Cl2, 2 equiv. of DBU, the one-pot tandem reaction/aminolysis sequence with morpholine proceeded smoothly at ambient temperature to afford the corresponding dienamide 5a in 73% yield.
|
|
Table 1 Optimization of reaction conditions.a |
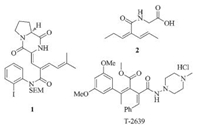
The scope of the tandem reaction was examined with various amines and trifluoromethyl ketones outlined in Table 2. Several secondary alkylamines, cyclic or linear, were found to be viable amine reagents, providing dienamide products with moderate to good isolated yields (Table 2, entries 1–5). More sterically demanding dibutylamine still delivered target, albeit with a diminished yield. N-Methyl or Ts aniline, with less nucleophilicity in comparison to aliphatic counterpart, was ineffective under the typical reaction conditions (some intractable polar compounds were observed). In addition, using morpholine or diethylamine, a range of different aryl substituted ketones competently engaged in the one-pot tandem reaction (Table 2, entries 6–14). The presence of electron-donating or -withdrawing substituents at different position of phenyl ring in CF3 ketones seemingly had a negligible influence on the chemical yield, and a range of densely functionalized dienamides were obtained in modest yields generally.
|
|
Table 2 Substrate scope of the one-pot reaction.a |
Furthermore, the isolated butenolide intermediate can also couple with simple allenoate via a conventional phosphine catalyzed [3 +2] cycloaddition, affording bicyclic lactones with moderate yield and E/Z selectivity (Eq. (1)).
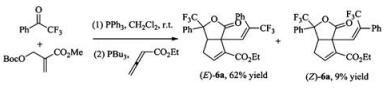
|
(1) |
A plausible reaction mechanism is proposed as shown in Scheme 2. Initially, the nucleophilic phosphine converts MBH carbonate to allylic phosphorus ylide via a well-defined SN2' addition/deprotonation process. The zwitterion A undergoes γ-addition/lactonization/deprotonation/Wittig olefination successive transformations to furnish butenolide intermediate. Amidines, as non-nucleophilic bases, have also been shown to function as nucleophilic catalysts in a wide range of reactions (including amidations)[18]. The nucleophilic DBU displaces the alkoxy moiety of butenolide to generate N-acyl DBU intermediate B, which undergoes the addition-elimination process with secondary alkylamine to afford dienamide 6. Alternatively, nucleophilic secondary alkylamines would react with lactone intermediate directly to furnish aminolysis product using DBU as a nonnucleophilic base.
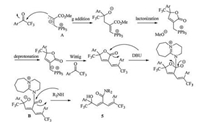
|
Download:
|
| S2. A plausible mechanism. | |
3. Conclusion
In conclusion, we have developed a one-pot protocol involving sequential phosphine-promoted tandem reaction and DBU-promoted amidation reaction, which afford trifluoromethylated dienamides possessing tertiary alcohol moiety in moderate to good yields at room temperature. This method provides a costeffective and step-economical approach to access trifluoromethylated dienamides with structural complexity from readily accessible reagent and starting materials under ambient and metal-free reaction conditions.
4. ExperimentalThe allylic carbonate 4 was prepared according to a known procedure [16c]. Amine, CF3 ketones and base reagents used in this work were purchased from commercial suppliers and were used without further purification. 1H, 19F and 13C NMR were recorded on Bruker 600 MHz spectrometers in CDCl3 solution, using TMS as an internal standard. HRMS were performed using electrospray ionization (ESI) with Waters Synapt G2 Si.
General procedure for the preparation of dienamide products: Triphenylphosphine (0.05 mmol) was added to a stirred solution of trifluoromethyl ketone 3 (0.2 mmol) and carbonate 4 (0.05 mmol) in CH2Cl2 (1 mL) in one portion. The resulting mixture was magnetically stirred at r.t. and monitored by TLC. After the reaction was complete (around 8 h), DBU (0.1 mmol) and alkylamines (0.2 mmol) was added sequentially and stirred for another 10 h. After completion of reaction as indicated by TLC, the crude mixture was concentrated and directly purified by flash column chromatography (petroleum ether/EtOAc, 3:1-1:1) to give the desired product 5 with indicated yields.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21302034), and the Fundamental Research Funds for the Central Universities (No. 2013HGQC0028).
| [1] | O. Saku, H. Ishida, E. Atsumi, et al., 5-diarylpentadienamides as orally available transient receptor potential vanilloid 1(TRPV1) antagonists. J. Med. Chem. 55 (2012) 3436–3451. DOI:10.1021/jm300101n |
| [2] | S.P. Reddy, Y. Venkateswarlu. First stereoselective synthesis of cytotoxic (-)-kunstleramide. Tetrahedron Lett. 54 (2013) 4617–4619. DOI:10.1016/j.tetlet.2013.06.034 |
| [3] | B. Yu, X.J. Shi, J. l. Ren, et al., Efficient construction of novel D-ring modified steroidal dienamides and their cytotoxic activities. Eur. J. Med. Chem. 66 (2013) 171–179. DOI:10.1016/j.ejmech.2013.05.035 |
| [4] | L.E. Overman, M.D. Rosen. Terminating catalytic asymmetric Heck cyclizations by stereoselective intramolecular capture of η-allyl palladium intermediates: total synthesis of (-)-spirotryprostatin B and three stereoisomers. Tetrahedron 66 (2010) 6514–6525. DOI:10.1016/j.tet.2010.05.048 |
| [5] | G.R. Granneman, S.I. Wang, J.M. Machinist, J.W. Kesterson. Aspects of the metabolism of valproic acid. Xenobiotica 14 (1984) 375–387. DOI:10.3109/00498258409151426 |
| [6] | H. Miyazaki, H. Sai, H. Ohmizu, et al., Synthesis and evaluation of 14-diphenylbutadiene derivatives as inhibitors of plasminogen activator inhibitor-1(PAI-1) production. Bioorgan. Med. Chem. 18 (2010) 1968–1979. DOI:10.1016/j.bmc.2010.01.032 |
| [7] |
(a) G. Magueur, B. Crousse, S. Charneau, et al. , Fluoroartemisinin: trifluoromethyl analogues of artemether and artesunate, J. Med. Chem. 47(2004) 2694-2699; (b) H. Schäcke, A. Schottelius, W. -D. Döcke, et al. , Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects, Proc. Natl. Acad. Sci. U. S. A. 101(2004) 227-232. |
| [8] |
(a) J. Nie, H. C. Guo, D. Cahard, J. A. Ma, Asymmetric construction of stereogenic carbon centers featuring a trifluoromethyl group from prochiral trifluoromethylated substrates, Chem. Rev. 111(2011) 455-529; (b) K. Yearick, C. Wolf, Catalytic enantioselective addition of diethylzinc to trifluoromethyl ketones, Org. Lett. 10(2008) 3915-3918; (c) R. Motoki, M. Kanai, M. Shibasaki, Copper(Ⅰ) alkoxide-catalyzed alkynylation of trifluoromethyl ketones, Org. Lett. 9(2007) 2997-3000. |
| [9] | S.M. ShakilHussain, R. Suleiman, B. El Ali. New conjugated dienamides via palladium-catalyzed selective aminocarbonylation of enynes. Tetrahedron Lett. 53 (2012) 6535–6539. DOI:10.1016/j.tetlet.2012.09.084 |
| [10] | R. Ding, Y. Li, C. Tao, B. Cheng, H. Zhai. Stereoselective synthesis of (2Z)-2, 4-dienamides via NBS-mediated allyloxyl addition-claisen rearrangement-dehydrobromination cascade reaction of ynsulfonamides. Org. Lett. 17 (2015) 3994–3997. DOI:10.1021/acs.orglett.5b01859 |
| [12] | S. Pachali, C. Hofmann, G. Rapp, et al., Stereoselective synthesis of (2E, 4Z)-dienamides employing (triphenylphosphoranylidene)ketene. Eur. J. Org. Chem (2009) 2828–2835. |
| [13] | B.R. D'Souza, J. Louie. Nickel-catalyzed cycloadditive couplings of enynes and isocyanates. Org. Lett. 18 (2009) 4168–4171. |
| [14] | C. Wang, J. Lu, G. Mao, Z. Xi. Preparation and electrophilic cyclization of multisubstituted dienamides leading to cyclic iminoethers. J. Org. Chem. 70 (2005) 5150–5156. DOI:10.1021/jo050433q |
| [15] |
(a) L. Liang, Y. Huang, Phosphine-catalyzed [3+3]-domino cycloaddition of ynones and azomethineimines to construct functionalized hydropyridazinederivatives, Org. Lett. 18(2016) 2604-2607; (b) H. Murayama, K. Nagao, H. Ohmiya, M. Sawamura, Phosphine-catalyzed vicinal acylcyanation of alkynoates, Org. Lett. 18(2016) 1706-1709; (c) Q. -F. Zhou, K. Zhang, L. Cai, O. Kwon, Phosphine-catalyzed intramolecular cyclizations of α-nitroethylallenoates forming (Z)-furanone oximes, Org. Lett. 18(2016) 2954-2957; (d) G. Zhan, M. L. Shi, Q. He, et al. , Catalyst-controlled switch in chemo-and diastereoselectivities: annulations of Morita-Baylis-Hillman carbonates from isatins, Angew. Chem. Ed. 55(2016) 2147-2151; (e) Y. N. Gao, F. C. Shi, Q. Xu, M. Shi, Enantioselective synthesis of isoquinolines: merging chiral-phosphine and gold catalysis, Chem. Eur. J. 22(2016) 6803-6807; (f) X. Han, W. L. Chan, W. Yao, et al. , Phosphine-mediated highly enantioselective spirocyclization with ketimines as substrates, Angew. Chem. Int. Ed. 55(2016) 6492-6496. |
| [16] |
(a) J. Ma, Z. Yuan, X. Kong, et al. , Reagent-controlled tandem reactions of vinyl epoxides: access to functionalized γ-butenolides, Org. Lett. 18(2016) 1450-1453; (b) H. Y. Duan, J. Ma, Z. Z. Yuan, et al. , Phosphine-promoted [3+ 2] cycloaddition between nonsubstituted MBH carbonates and trifluoromethyl ketones, Chin. Chem. Lett. 26(2015) 646-648; (c) H. Xiao, H. Duan, J. Ye, et al. , Chemoselective synthesis of trifluoromethylated γ-butenolide derivatives via phosphine-promoted tandem reaction of allylic carbonates and trifluoromethyl ketones, Org. Lett. 16(2014) 5462-5465; (d) H. Xiao, Z. Chai, R. S. Yao, G. Zhao, Phosphine catalyst-controlled cycloaddition or dienylation reactions of trifluoromethyl aryl ketones with bis-substituted allenoates, J. Org. Chem. 78(2013) 9781-9790. |
| [17] |
(a) K. E. Price, C. Larrivée-Aboussafy, B. M. Lillie, et al. , Mild and efficient DBUcatalyzed amidation of cyanoacetates, Org. Lett. 11(2009) 2003-2006; (b) J. E. Taylor, M. D. Jones, J. M. J. Williams, et al. , Friedel-Crafts acylation of pyrroles and indoles using 1, 5-diazabicyclo[4. 3. 0]non-5-ene (DBN) as a nucleophilic catalyst, Org. Lett. 12(2010) 5740-5743; (c) T. Ohshima, Y. Hayashi, K. Agura, et al. , Sodium methoxide: a simple but highly efficient catalyst for the direct amidation of esters, Chem. Commun. 48(2012) 5434-5436; (d) L. Yang, D. Shi, S. Chen, et al. , Microwave-assisted synthesis of 2, 3-dihydropyrido[2, 3-d]pyrimidin-4(1H)-ones catalyzed by DBU in aqueous medium, Green Chem 14(2012) 945-951; (e) H. Morimoto, R. Fujiwara, Y. Shimizu, et al. , Lanthanum(Ⅲ) triflate catalyzed direct amidation of esters, Org. Lett. 16(2014) 2018-2021; (f) N. Caldwell, C. Jamieson, I. Simpson, A. J. B. Watson, Catalytic amidation of unactivated ester derivatives mediated by trifluoroethanol, Chem. Commun. 51(2015) 9495-9498. |
| [18] | J.E. Taylor, S.D. Bull, J.M.J. Williams. Amidines, isothioureas, and guanidines as nucleophilic catalysts. Chem. Soc. Rev. 41 (2012) 2109–2121. DOI:10.1039/c2cs15288f |
 2017, Vol. 28
2017, Vol. 28