b State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
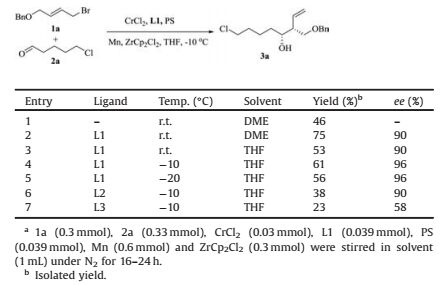
The 1, 3-diol is a basic framework found in numerous natural products and organic materials (Fig. 1) [1-5]. It is also an important kind of synthetic intermediates that exist in a wide range of organic transformations. Therefore, 1, 3-diol subunits have been employed as the valuable building block for the synthesis of various natural products and biologically active compounds [6-11]. Quite a few synthetic methods have been developed to construct the 1, 3-diol core skeleton [12]. Recently, transition-metal-catalyzed reactions (Pd, In and SnCl2) were applied to the preparation of 1, 3-diol derivatives from various starting substrates (such as 2-butene-1, 4-diol carboxylates or vinyl epoxides) [12a, 12b, 12c]. Among these transition-metals, the chromium-catalyzed protocols are attractive approach due to its high chemoselectivity and excellent compatibility with various functional groups. In 2004, Cozzi and Umani-Ronchi reported the chromium-catalyzed Nozaki-HiyamaKishi (NHK) reaction to synthesize 1, 3-diols derivatives with moderate diastereo-and enantioselectivity favoring syn 1, 3-diols using salen ligands [13, 14].
|
Download:
|
| Fig. 1. Representative natural products containing 1, 3-diols. | |
Given the wide occurrence of this type of molecular skeleton in natural products and biologically active compounds, further improvements for preparation of 1, 3-diols derivatives with high enantioselectivity under mild reaction conditions are highly desired. In the past two years, we have been interested in chromium-catalyzed reactions and have focused on the development of carbazole-based bisoxazolines in asymmetric transformations [15-17]. As a continuation of our previous research, herein we reported a highly enantioselective chromium-catalyzed allylation of aldehydes to provide 1, 3-diols derivatives under mild reaction conditions. This method provides a complementary and a more practical strategy for the synthesis of 1, 3-diols with tolerance of a broad range of functional groups.
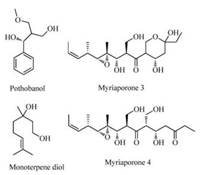
2. Results and discussionSeveral kinds of chiral ligands have been successfully identified to induce high stereoselectivity in chromium-catalyzed asymmetric transformations [18, 19]. Carbazole-based chiral ligands developed by Nakada, were used in asymmetric chromium-catalyzed allylation of various aldehydes with high enantioselectivity [20]. Recently, our group developed a more convergent approach to synthesize this type of ligands (Fig. 2) [15a].
|
Download:
|
| Fig. 2. Ligands screened in NHK reactions. | |
Initially, we tested the reaction of (E)-(((4-bromobut-2-en-1-yl) oxy)methyl)benzene (1a) and 5-chloropentanal (2a) with 10 mol% of chromium(Ⅱ) chloride in DME at room temperature, and the desired product 3a was obtained in 46% yield (Table 1, entry 1). Delightfully, when the chiral carbazole ligand (L1) was used, the product 3a was obtained in even better yield (75%) and high enantioselectivity (90% ee) (Table 1, entry 2). Solvent screening showed that THF was better than DME (Table 1, entry 3). It was found that the temperature is an important influential factor for the enantioselectivity of this reaction, when the reaction temperature lowered down to -10 ℃, the product 3a was obtained in 96% ee (Table 1 entry 4). The temperature lowered further to -20 ℃, the enantioselectivity remained the same but the yield decreased (56%) (Table 1, entry 5). The effects of a variety of chiral ligands in the reaction system were also tested. When ligand L2 and L3 were used as ligands in this reaction, comparable and moderate enantioselectivities were obtained, however, the yields were decreased to 38% and 23% respectively (Table 1, entries 6-7).
|
|
Table 1 Optimization of the reaction conditionsa. |
With the optimized reaction conditions in hand, the substrate scope was promptly investigated. Firstly, we checked the reaction of (E)-(((4-bromobut-2-en-1-yl)oxy)methyl)benzene (1a) with various aldehydes; the representative aliphatic aldehydes, such as capronaldehyde and 3-phenylpropanal, reacted efficiently under this reaction conditions and the products 3b and 3c were obtained with high enantioselectivity (93% and 95% ee) and in moderate yields (Table 2). It was noted that naturally occurring aldehyde, citronellal was also a good substrate under this reaction condition and gave 3d in 87% yield with 95% ee. Aromatic aldehydes, such as benzaldehyde and 3-fluoro-benzaldehyde, reacted with 1a to afford the products 3e and 3f with 90% and 91% ee. Reactions of the cinnamaldehyde and its derivatives with electron-withdrawing groups and electron-donating groups on the aromatic ring also proceeded smoothly to furnish a range of desired 1, 3-diol products 3g-3i in moderate yield with high enantioselectivity (96%-98% ee). 2-Thenaldehyde was converted to 3j under the standard condition to give a good isolated yield (73%) and a high enantioselectivity (91% ee). We were pleased to find that benzyl protection could be replaced with TBDPS group. The coupling of the latter with four aliphatic aldehydes proceeded equally well in terms of efficiency and enantioselectivity, as the corresponding 3k-3n were obtained with high ee values and in comparable yields.
|
|
Table 2 Substrate scope studies a, b. |
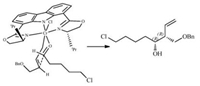
The stereochemistry of products was assigned by comparison with the reported data of stereoisomer of 3e [13]. Relying on a proposed working transition model, the configuration of the product 3a (>97% ee) was tentatively assigned to be (R, R) (Fig. 3).
|
Download:
|
| Fig. 3. Proposed transition state. | |
3. Conclusion
In summary, an efficient and highly enantioselective method for the preparation of 1, 3-diols through chromium-catalyzed carbonyl allylation with protected (E)-4-bromobut-2-en-1-ol is reported. A carbazole-based bisoxazoline serves as a universal chiral ligand. The protocol established in this study constitutes a more efficient and a practical strategy for the synthesis of 1, 3-diols derivatives with tolerance of a variety of functional groups under mild reaction conditions. The future work will focus on applying this catalytic system to the synthesis of complex molecules with bioactivities.
4. ExperimentalAn experimental procedure includes as the following: (1) A complexation step in which a suspension of CrCl2 in THF was treated with chiral ligands and proton sponges at room temperature to in situ generate the corresponding chromium complex; (2) An allylation step in which the catalyst solution was transferred to a mixture of Mn, ZrCp2Cl2, followed by the addition of 2 and 1 at -10 ℃. After 24 h, the reaction solution was purified and the desired product 3 was obtained.
AcknowledgmentWe are grateful to NSFC (Nos. 21421091, XDB20000000), the "Thousand Plan" Youth program for financial support.
| [1] |
(a) Y. Zhou, P. Prediger, L.C. Dias, A.C. Murphy, F. Leadlay, Macrodiolide formation by the thioesterase of a modular polyketide synthase, Angew. Chem. Int. Ed. 54(2015) 5232-5324; (b) J.J. Feng, J. Victory, J. Garza, M.J. Krische, Redox-triggered C-C coupling of alcohols and vinyl epoxides:diastereo-and enantioselective formation of allcarbon quaternary centers via tert-(hydroxy)-prenylation, J. Am. Chem. Soc. 136(2014) 8911-8914; (c) C. Daniel, R. Schmitt, A. Marie, D. Schmitt, M.J. Krische, Iridium-catalyzed allylation of chiral-stereogenic alcohols:bypassing discrete formation of epimerizable aldehyde, Org. Lett. 14(2012) 6302-6305; (d) L.M. Geary, M.J. Krische, Successive C-C coupling of dienes to vicinally dioxygenated hydrocarbons:ruthenium catalyzed[4+2] cycloaddition across the diol, hydroxycarbonyl, or dione oxidation levels, J. Am. Chem. Soc. 135(2013) 3796-3799. |
| [2] | M. Pérez, C. del Pozo, F. Reyes. Total synthesis of natural myriaporones. Angew. Chem. Int. Ed. 43(2004)1724–1727. DOI:10.1002/(ISSN)1521-3773 |
| [3] | R.E. Taylor, K.N. Fleming. Total synthesis and stereochemical assignment of myriaporones 13. and 4. Angew. Chem. Int. Ed. 43(2004)1728–1730. DOI:10.1002/(ISSN)1521-3773 |
| [4] | G. Ehrlich, J. Hassfeld, U. Eggert, M. Kalesse. The total synthesis of (+)-tedanolide. J. Am. Chem. Soc. 128(2006)14038–14039. DOI:10.1021/ja0659572 |
| [5] | A.B. Smith Ⅲ , D. Lee. Total synthesis of (+)-tedanolide. J. Am. Chem. Soc. 129(2007)10957–10962. DOI:10.1021/ja073329u |
| [6] | M. Abdul Muhit, H. Noguchi. Phenolic constituents of the bangladeshi medicinal plant pothos scandens and their anti-estrogenic, hyaluronidase inhibition, and histamine release inhibitory activities. Phytochemistry 121(2016)30–37. DOI:10.1016/j.phytochem.2015.10.009 |
| [7] | H. Knapp, P.J. Winterhalter. Agric (s)-3.7-dimethyl-5-octene-1, 7-diol and related oxygenated monoterpenoids from petals of rosa damascena mill. Food Chem. 46(1998)1966–1970. DOI:10.1021/jf970987x |
| [8] | L.B. Andre, S. Barreiros, J.M. David. A-type proanthocyanidin antioxidant from dioclea lasiophyll. Phytochmistry 55(2000)805–808. DOI:10.1016/S0031-9422(00)00297-1 |
| [9] | H. Matsuda, M. Yoshikawa. Inhibitors from the rhizomes of alpinia officinarum on production of nitric oxide in lipopolysaccharide-activated macrophages and the structural requirements of diarylheptanoids for the activity. Bioorg. Med. Chem. 14(2006)138–142. DOI:10.1016/j.bmc.2005.08.003 |
| [10] | V. Rukachaisirikul, J. Sakayaroj. Rare 2-phenylpyran-4-ones from these agrassderivedfungipolyporales PSU-ES44 and PSU-ES83. Tetrahedron 69(2013)6981–6986. DOI:10.1016/j.tet.2013.06.059 |
| [11] | A. Esmurziev, E. Sunby, B.H. Hoff. Regioselective c-6 hydrolysis of methylobenzoyl-pyranosides catalysed by candida rugosa lipase. Eur. J. Org. Chem (2009)1592–1597. |
| [12] |
(a) Y. Masuyama, J.P. Takahara, Y. Kurusu, Allylic alcohols as synthons of allylic carbanions, palladium-catalyzed carbonyl allylation by allylic alcohols with tin dichloride, J. Am. Chem. Soc. 110(1988) 4473-4474; (b) J.P. Takahara, Y. Masuyama, Y. Kurusu, Palladium-catalyzed carbonyl allylation by allylic alcohols with stannous chloride, J. Am. Chem. Soc. 114(1992) 2577-2586; (c) X.L. Wang, Y.Y. Yang, H.J. Chen, Y. Wu, D.S. Ma, Synthesis of vinylchorinecontaining 1, 3-diols from marine cyanophyte, Tetrahedron 70(2014) 4571-4579; (d) S. Araki, K. Kameda, J. Tanaka, et al., Umpolung of vinyloxiranes:regio-and stereoselectivity of the In/Pd-mediated allylation of carbonyl compounds, J. Org. Chem. 66(2001) 7919-7921; (e) M. Gagliardo, N. Selander, N.C. Mehendale, et al., Catalytic performance of symmetrical and unsymmetrical sulfur-containing pincer complexes:synthesis and tandem catalytic activity of the first PCS-pincer palladium complex, Chem. Eur. J. 14(2008) 4800-4809. |
| [13] | M. Bandini, G. Cozzi, S. A. Licciulli. Umani-Ronchi. A cross metathesis based protocol for the effective synthesis of functionalised allyl bromides and chlorides. Synthesis 3(2004)409–414. |
| [14] | M. Lombardo, S. Licciulli, S. Morganti, C. Trombini. Trombini C.. 3-Chloropropenyl pivaloate in organic synthesis:the first asymmetric catalytic entry to syn-alk-1-ene-3. 4-diols. Chem. Comm (2003)1762. |
| [15] |
(a) W. Chen, Q. Yang, T. Zhou, Q. Tian, G. Zhang, Enantioselective synthesis of α-exo-methyleneg-butyrolactones via chromium catalysis, Org. Lett.17(2015) 5236-5239; (b) Q. Tian, G. Zhang, Recent advances in the asymmetric Nozaki-Hiyama-Kishi reaction, Synthesis 48(2016) 4048-4049. |
| [16] | Q. Tian, B. Bai, B. Chen, G. Zhang. Chromium-catalyzed asymmetric dearomatization addition reactions of halomethyl heteroarenes. Org. Lett. 18(2016)1828–1831. DOI:10.1021/acs.orglett.6b00559 |
| [17] | Y. Xiong, G. Zhang. Enantioselective synthesis of quaternary stereocenters via chromium catalysis. Org. Lett. 18(2016)5094–5097. DOI:10.1021/acs.orglett.6b02540 |
| [18] |
(a) G.C. Hargaden, P.J. Guiry, The development of the asymmetric NozakiHiyama-Kishi reaction, Adv. Synth. Catal. 349(2007) 2407; (b) K. Sugimoto, S. Aoyagi, C. Kibayashi, Enantioselective allylation of aldehydes with (dialkoxyallyl) chromium(Ⅲ) complexes, J. Org. Chem. 62(1997) 2322-2323; (c) M. Bandini, P.G. Cozzi, P. Melchiorre, A. Umani-Ronchi, The first catalytic enantioselective nozaki-hiyama reaction, Angew. Chem. Int. Ed. 38(1999) 3357-3359; (d) A. Berkessel, D. Menche, C.A. Sklorz, M. Schroöder, I. Paterson, A highly enantioselective catalyst for the asymmetric nozaki-hiyama-kishi reaction of allylic and vinylic halides, Angew. Chem. Int. Ed. 42(2003) 1062-1065; (e) C. Chen, K. Tagami, Y. Kishi, Ni(Ⅱ)/Cr(Ⅱ)-mediated coupling reaction:an asymmetric process, J. Org. Chem. 60(1995) 5386-5387; (f) H.W. Choi, D. Demeke, F.A. Kang, et al., Synthetic studies on the marine natural product halichondrins, Pure Appl. Chem. 75(2003) 1-17; (g) H.B. Guo, C.G. Dong, D.S. Kim, et al., Toolbox approach to the search for effective ligands for catalytic asymmetric Cr-mediated coupling reactions, J. Am. Chem. Soc. 131(2009) 15387-15393. |
| [19] |
(a) Y. Okude, S. Hirano, T. Hiyama, H. Nozaki, Grignard-type carbonyl addition of allyl halides by means of chromous salt. A chemospecific synthesis of homoallyl alcohols, J. Am. Chem. Soc. 99(1977) 3179-3181; (b) K. Takai, K. Kimura, T. Kuroda, T. Hiyama, H. Nozaki, Selective grignard-type carbonyl addition of alkenyl halides mediated by chromium (Ⅱ) chloride, Tetrahedron Lett. 24(1983) 5281-5284; (c) H. Jin, J.I. Uenishi, W.J. Christ, Y. Kishi, Catalytic effect of nickel(Ⅱ) chloride and palladium(Ⅱ) acetate on chromium(Ⅱ)-mediated coupling reaction of iodo olefins with aldehydes, J. Am. Chem. Soc. 108(1986) 5644-5646; (d) K. Takai, M. Tagashira, T. Kuroda, et al., Reactions of alkenylchromium reagents prepared from alkenyl trifluoro-methanesulfonates (triflates) with chromium(Ⅱ) chloride under nickel catalysis, J. Am. Chem. Soc. 108(1986) 6048-6050; (e) M. Avalos, R. Babiano, P. Cintas, J.L. Jimenez, J.C. ' Palacios, Synthetic variations based on low-valent chromium:new developments, Chem. Soc. Rev. 28(1999) 169-177. |
| [20] |
(a) M. Inoue, T. Suzuki, M. Nakada, A new asymmetric tridentate carbazole ligand:its preparation and application to Nozaki-Hiyama allylation, Synlett 4(2003) 570-572; (b) M. Inoue, T. Suzuki, M. Nakada, Asymmetric catalysis of Nozaki-Hiyama allylation and methallylation with a new tridentate bis (oxazolinyl) carbazole ligand, J. Am. Chem. Soc. 125(2003) 1140-1141. |
 2017, Vol. 28
2017, Vol. 28