b State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361005, China;
c Key Laboratory of Global Change and Marine-Atmospheric Chemistry, Third Institute of Oceanography, State Oceanic Administration, Xiamen 361005, China
Carbon dots, generally small carbon nanoparticles less than 10 nm in size, have received extensively attention due to their excellent properties, such as low toxicity, favorable biocompatibility, stable photoluminescence and good solubility [1-4]. These fascinating merits make them widely used in the areas of physical/chemical sensors, fuel cells, photocatalytic devices and bioimaging aspect [5-12]. Most of the carbon dots emit blue fluorescence, which is unfavorable in bioimaging and insensitive to visual observation. Functionalization, mainly post-surface-modification and intrinsic heteroatom doping, are efficient ways to tune the chemical and physical properties of carbon dots [13]. Post-surfacemodification is tedious and the stability of carbon dots is a major problem in the following applications. Intrinsic heteroatom doping is more popular. Incorporating metal ions and transition metal ions into the core of the carbon dots provides more conjugated intrinsic defects, resulting in the red shift of their fluorescence. However, metal and transition metal atoms are too much larger than carbon atom. This produces the ineffective and heterogeneous doping. In addition, the toxicity of metal ions is also a big issue. Inversely, both boron and nitrogen atoms are comparable with carbon atom in size, they can form into uniform doping and homogeneous intrinsic defects. The synthesis process is simpler, more effective and convenient. Recently, co-doped multiple heteroatom has begun to gain extensive attention because it can create a unique electronic structure owing to the synergistic effect between the doped heteroatom [14]. Nevertheless, to date, N, S-co doped and N, P-co doped carbon dots [15-17] have synthesized highly fluorescent carbon dots with high quantum yield (QY). However, most of them emit blue fluorescence. Though there was a serious of good results with reported B, N co-doped for chemical sensing using hydrothermal method [18, 19], most of the B, N doped carbon nanomaterials were used for catalysis [20]. Therefore, the aim of this work is to tune the fluorescence properties of B, N-carbon dots and extend their application in chemical sensing.
Copper is a widely used industrial metal, with the rapid industrial development, environmental pollution has become a serious problem, the detection of Cu2+ is a significant issue in environmental monitoring [21, 22]. Currently, various fluorescent materials have been developed for the determination of Cu2+, including small organic molecules [23, 24], noble metal nanoclusters [25] and semiconductor quantum dots [26]. However, most of the traditional fluorescent materials suffer the drawbacks such as high toxicity, vulnerable to photo bleaching, low stability and tedious post-treatment. Thus, it is still essential to develop carbon dots based fluorescence materials toward the detection of Cu2+ with lower toxicity, better photostability and simper posttreatment.
Herein, fluorescent B, N-carbon dots were obtained via a combustion method with aminophenylboronic acid (APBA) as the raw material. The as-prepared B, N-carbon dots emitted green fluorescence with a QY of 1.6%. They revealed high resistance to photobleaching and ionic strength. Using the B, N-carbon dots as the fluorescence probe, a facile fluorescence sensing approach for Cu2+ detection was developed via static fluorescence quenching. Finally, the proposed method showed its applicability to natural water samples spiked with Cu2+.
2. Results and discussion 2.1. Characterization of the B, N-carbon dotsAminophenylboronic acid was chosen as the precursor because it contains abundant boron, nitrogen elements and has a stable aromatic structure. The as-prepared B, N-carbon dots remains transparent without any aggregation for 12 months at room temperature, indicating their excellent stability. The morphology of the B, N-carbon dots was characterized by TEM. As shown in Fig. 1A, the B, N-carbon dots were homodispersed in a narrow diameter ranging 1.5-4.0 nm, with a mean size around 2.5 nm. To further explore the composition and structure of the B, N-carbon dots, FT-IR and XPS investigation were conducted (Fig. 1B-F), the B, N-carbon dots exhibited the characteristic peaks of the raw material (APBA). There were B-C bond (the absorption band at 1100 cm-1), B-N bond (1390 cm-1), C-O, C=O bonds (1643 cm-1) and B-NH, B-OH bonds (3420-3500 cm-1) within the B, N-carbon dots [27, 28], which implied that both the boron and the nitrogen elements have been doped into the B, N-carbon dots. The XPS spectra showed C, N, B, O four elements with a C/N/O/ B concentration ratio of 0.612/0.059/0.273/0.056, the boron and nitrogen contents are rather higher than the relative reported literatures [29, 30]. The C 1s spectrum showed five peaks, centered at 283.9, 284.6, 285.2, 286.2 and 288.0 eV, attributed to C-B bond, C-C, C=C bonds, C-N bond, C-O bond and C=N bond, respectively. The N 1s spectrum showed two peaks, centered at 399.5 and 401.5 eV, corresponded to C-N bond and C-NH bond. The B 1s spectrum showed two peaks, centered at 190.8 and 191.7 eV, ascribed to B-C bond and B-O bond [5, 21, 31-34]. All the above results confirmed that the boron and the nitrogen elements were doped into the B, N-carbon dots.

|
Download:
|
| Figure 1. (A) TEM image of the B, N-carbon dots. Inset: the corresponding size distribution of the B, N-carbon dots. (B) FI-IR spectra and (C) XPS spectra of APBA and the B, Ncarbon dots. (D) C 1s spectrum, (E) N 1s spectrum and (F) B 1s spectrum of the B, N-carbon dots. | |
2.2. Optical properties of the B, N-carbon dots
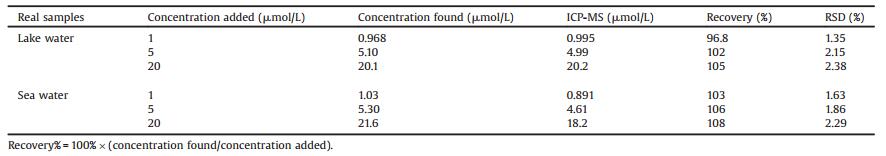
The optical properties of the B, N-carbon dots were characterized by UV-vis absorption and fluorescence emission spectra. As displayed in Fig. 2A, the B, N-carbon dots showed two unconspicuous absorption bands at 230 and 267 nm. The former might be ascribed to the π-π* absorption of the C=C core, the latter might originate from C=N, C=O functional groups [35]. The maximum fluorescence excitation and emission wavelengths were 310 and 520 nm. The large stokes shift and intensive green fluorescence of the B, N-carbon dots (inset photo in Fig. 2A) indicated their potential application in bioimaging. The fluorescence of the B, Ncarbon dots was excitation independent, the fluorescence emission wavelength kept constant at 520 nm under excitation from 300 to 390 nm (Fig. 2B), which confirmed the homogenous structure of the B, N-carbon dots. Using quinine sulfate as the reference, the QY of the B, N-carbon dots was estimated to be 1.6% (Table S1 in Supporting information). The pH value had an obvious effect on the fluorescence intensity of the B, N-carbon dots. The fluorescence intensity of the B, N-carbon dots firstly gradually increased at pH from 2 to 7, and then drops gradually from 7 to 12 (Fig. 2C), which might be ascribed to the considerable quantity of carboxyl and amino groups on their surface. The optimal pH is 7, so the neutral condition was chosen in the following experiments. As shown in Fig. 2D and S1 (Supporting information), the B, N-carbon dots exhibited high resistance to light illumination and high ionic strength, fluorescence intensity of the B, N-carbon dots remained constant after continuous irradiation for 2 h and in 1 mol/L NaCl solution. These results indicated the potential application of the B, N-carbon dots in fluorescence imaging under physiological conditions.
|
Download:
|
| Figure 2. (A) UV–vis absorption, fluorescence excitation and emission spectra of the B, N-carbon dots. Inset: photographs of the B, N-carbon dots solution under daylight (left) and 365 nm UV light (right). (B) The emission spectra of the B, N-carbon dots under different excitation wavelengths ranging from 300 to 390 nm. (C) Fluorescence responses of the B, N-carbon dots in different pH solutions (10 mmol/L PBS). (D) Stability of the B, N-carbon dots in the presence of different concentrations of NaCl. | |
2.3. Fluorescence detection of the B, N-carbon dots toward Cu2+
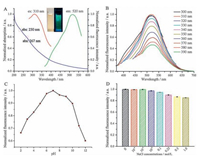
The abundant carboxyl, hydroxyl and amino groups on the surface of the B, N-carbon dots endowed their affinity to metal ions. Therefore, the feasibility of the B, N-carbon dots for Cu2+ was investigation. As shown in Fig. 3A, the fluorescence intensity of the B, N-carbon dots led to a quick (2 min) and obvious decrease with the addition of Cu2+. In the determination of Cu2+, the fluorescence intensity of the B, N-carbon dots gradually decreased with the increase of Cu2+ concentration, and showing a good linearity from 1 to 25 μmol/L toward Cu2+ detection, with a detection limit of 0.3 μmol/L at a signal-to-noise ratio of 3 (Fig. 3B and C). The fluorescence intensity versus the concentration of Cu2+ could be fitted into a linear equation F0/F=0.0573[Cu2+] (μmol/L) + 0.894, with a correlation coefficient R of 0.994. Similar fluorescent methods toward the determination of Cu2+ are listed in Table S2. Though the detection limit of the proposed method is not the most sensitive one, according to the US Environmental Protection Agency, the maximum concentration of Cu2+ in drinking water should be no more than 20 μmol/L [21]. Satisfyingly, the proposed approach is sensitive enough to detection Cu2+ in drinking water.
|
Download:
|
| Figure 3. (A) Kinetic characteristic of the fluorescence response of the B, N-carbon dots to 25 μmol/L Cu2+. (B) Fluorescence responses of the B, N-carbon dots in the presence of increasing concentrations of Cu2+ in 10 mmol/L PBS (pH 7.0). (C) The relationship between F0/F and the concentration of Cu2+. Inset: the linear response to the concentration of Cu2+ ranging from 1 to 25 μmol/L. (D) Fluorescence responses of the B, N-carbon dots in 25 μmol/L various ions. F0 and F represent the fluorescence intensities of the B, Ncarbon dots in the absence and presence of Cu2+ or other ions. | |
To evaluate the selectivity of the B, N-carbon dots toward Cu2+ detection, a variety of co-existing ions were investigated to their effects on fluorescence intensity of the B, N-carbon dots. As displayed in Fig. 3D, the influences were ignorable within the linearity range. The application of Cu2+ detection approach was tested in natural water samples as well. As shown in Table 1, the recoveries ranged from 96.8% to 108%, and the results matched well with those obtained from ICM-MS (the differences in sea water may be ascribed to influences of salinity), suggesting the potential applicability of the proposed method to natural water samples. The concentrations of Cu2+ in the lake and sea water were 0.0171 and 0.0630 μmol/L, which were beyond the detection limit of the proposed method. These results revealed that the water of Xiamen is relatively clean.
|
|
Table 1 Determination of Cu2+ spiked in real samples (n=3). |
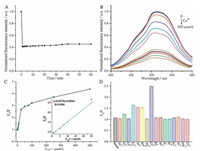
To explore the detection mechanism of the B, N-carbon dots toward Cu2+ detection, the fluorescence lifetime of the B, N-carbon dots with and without 25 μmol/L Cu2+ was investigated. As shown in Fig. 4A, the lifetimes of the B, N-carbon dots kept the same under an excitation of 297 nm. The fluorescence decay was fitted using a three-exponential decay function to yield a lifetime of 2.72 ns. This result excluded the possibility of the dynamic fluorescence quenching process, and the possible detection mechanism is static fluorescence quenching process. Additionally, the absorption spectra of the B, N-carbon dots with respect to the concentration of Cu2+ were collected. As shown in Fig. 4B, a new absorption band at 256 nm with the addition of Cu2+ was noted, which might be attributed to the binding of Cu2+ to the nitrogen atom on the B, N carbon dots (form Cu2+-B, N-carbon dots complex). These further confirmed that the determination of Cu2+ was induced by the static fluorescence quenching.
|
Download:
|
| Figure 4. (A) Time-resolved decay of the B, N-carbon dots with and without 25 μmol/L Cu2+ in 10 mmol/L PBS (pH 7.0). (B) UV–vis absorption spectra of the B, N-carbon dots in the presence of 0 to 500 μmol/L Cu2+ in 10 mmol/L PBS (pH 7.0). Inset is: the relationship between the absorbance of the B, N-carbon dots at 250 nm and the concentration of Cu2+. | |
3. Conclusion
In this study, fluorescent B, N-carbon dots were synthesized by a combustion method. Using the B, N-carbon dots as the fluorescent probe, an effective and facile fluorescence approach was developed for the determination of Cu2+. Under optimal conditions, the detection of Cu2+ could be completed in 2 min with a detection limit of 0.3 μmol/L, and independent of other metal ions. In addition, the proposed approach showed potential application for the Cu2+ detection in natural water samples.
4. Experimental 4.1. MaterialsAminophenylboronic acid (APBA) was purchased from Sigma-Aldrich (USA). Ethyl alcohol (99.7%), nitric acid (68%), sulfuric acid (98%), all metal nitrates and chlorides were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were of analytical grade and used as received without further purification. All solutions were prepared using ultrapure water with a resistivity of 18.2 MΩ/cm from a Millipore purification system (Millipore, USA).
4.2. InstrumentationUV-vis absorption and fluorescence spectra were tested on a UV2550 spectrophotometer (Shimadzu, Japan) and an F-4500 spectrophotometer (Hitachi, Japan). Fourier transform infrared (FT-IR) spectra were performed on a Nicolet 330 spectrophotometer (Thermo Electron Corp., USA). The fluorescence lifetime was obtained with a FluoroMax-4 spectrofluorometer (Horiba JobinYvon, France). Transmission electron microscope (TEM) images were collected using a JEM-2100 (JEOL, Japan) at an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were carried on a PHI Quantum 2000 XPS system (Physical Electronics, USA) with Al Kα (hv=1486.60 eV). Inductively coupled plasma mass spectroscopy (ICP-MS) was performed using a 7500ce ICP-MS instrument (Agilent, USA).
4.3. Preparation of the B, N-carbon dotsIn a typical synthesis procedure for B, N-carbon dots (Scheme 1), 5 g APBA was added into 30 mL ethyl alcohol to form a transparent aqueous. Pour the precursor aqueous into an empty alcohol burner (50 mL), fix a 50 mL glass beaker inverted with an iron support on the right above the alcohol burner. Light the alcohol burner (with aqueous), black smoke began to form onto the inner wall of the glass beaker. After about 2 h, the inner wall of the glass beaker turns into black, covered with carbon dusk. Collect the carbon dusk with a scraper blade, the sum weight of the carbon dusk was 1.870 g. Weigh 2 mg of the carbon dusk, and add into a mixed acid (1 mL nitric acid and 3 mL sulfuric acid), reflux at 80 ℃ for 12 h, then neutralize the solution to pH 7.0, dialysis overnight (the molecular weight cutoff of the dialysis bag is 14, 000), finally collect the solution of B, N-carbon dots. The productivity is about 18.7%, and the concentration of as-prepared B, N-carbon dots is 10 μg/mL.
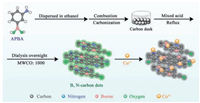
|
Download:
|
| Scheme 1. The synthesis of the B, N-carbon dots and their fluorescence detection for Cu2+. | |
4.4. Fluorescence sensing of Cu2+
100 μL of the as-prepared B, N-carbon dots were mixed with 10 mmol/L phosphate buffer solution (PBS, pH 7.0) containing different concentrations (0, 0.5, 1.0, 2.5, 7.5, 10, 25, 50, 75, 100, 250 and 500 μmol/L) of Cu2+. The final volume of the solution was kept at 1.0 mL. After 2 min of reaction at room temperature, the fluorescence spectra of the solution were collected in the wavelength from 400 to 600 nm (the excitation wavelength was 310 nm, both excitation and emission slits were 5 nm).
In the investigation of the selectivity of Cu2+, several coexisting metal ions were selected and the concentration of all ions was 25 μmol/L. The detection conditions were the same as mentioned above.
In the determination of Cu2+ in water samples, lake water samples were obtained in Xiamen University campus and the sea water samples were collected from the sea around Xiamen University. The water samples were first centrifuged at 12, 000 rpm for 30 min twice and then the supernatant was filtered through the 0.22 mm water phase membrane. 100 mL of the asprepared B, N-carbon dots were injected into 10 mmol/L PBS (pH 7.0) containing 0.5 mL water sample and different concentrations (1, 5 and 20 μmol/L) of Cu2+. The final volume of the solution was 1 mL. The detection conditions were the same as mentioned above. To avoid the influence of high salinity of sea water, the sea water was diluted ten times when detected using the ICM-MS.
Time-resolved decay measurements were applied to explore the reaction mechanism between the B, N-carbon dots and Cu2+. 100 mL of the B, N-carbon dots were added into10 mmol/L PBS (pH 7.0) containing with/without 25 μmol/L Cu2+. The final volume of the solution was 1mL. The excitation wavelength of the nanoled was 297nm (the closest to 310nm) and the emission wavelength was 520nm.
AcknowledgmentsThis research was financially supported by the National Natural Science Foundation of China (No. 21375112) and the Marine hightech industry development projects of Fujian Province(No.2015-19).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.009.
| [1] | Wang Y.F., Hu A.G.. Carbon quantum dots:synthesis, properties and applications. J. Mater. Chem.C 2 (2014) 6921–6939. DOI:10.1039/C4TC00988F |
| [2] | Baker S.N., Baker G.A.. Luminescent carbon nanodots:emergent nanolights. Angew. Chem. Int. Ed. 49 (2010) 6726–6744. DOI:10.1002/anie.200906623 |
| [3] | Li L.L., Wu G.H., Yang G.H., et al., Focusing on luminescent graphene quantum dots:current status and future perspectives. Nanoscale 5 (2013) 4015–4039. DOI:10.1039/c3nr33849e |
| [4] | Zheng X.T., Ananthanarayanan A., Luo K.Q., Chen P.. Glowing graphene quantum dots and carbon dots:properties, syntheses, and biological applications. Small 11 (2015) 1620–1636. DOI:10.1002/smll.v11.14 |
| [5] | Fan Z.T., Li Y.C., Li X.H., et al., Surrounding media sensitive photoluminescence of boron-doped graphene quantum dots for highly fluorescent dyed crystals, chemical sensing and bioimaging. Carbon 70 (2014) 149–156. DOI:10.1016/j.carbon.2013.12.085 |
| [6] | Lim S.Y., Shen W., Gao Z.Q.. Carbon quantum dotsand their applications. Chem. Soc. Rev. 44 (2015) 362–381. DOI:10.1039/C4CS00269E |
| [7] | Hong G.S., Diao S., Antaris A.L., Dai H.J.. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 115 (2015) 10816–10906. DOI:10.1021/acs.chemrev.5b00008 |
| [8] | Ding C.Q., Zhu A.W., Tian Y.. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc. Chem. Res. 47 (2014) 20–30. DOI:10.1021/ar400023s |
| [9] | Wang H.B., Maiyalagan T., Wang X.. Review on recent progress in nitrogendoped graphene:synthesis, characterization, and its potential applications. ACS Catal. 2 (2012) 781–794. DOI:10.1021/cs200652y |
| [10] | Guo Y.M., Zhang L.F., Zhang S.S., et al., Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens. Bioelectron. 63 (2015) 61–71. DOI:10.1016/j.bios.2014.07.018 |
| [11] | Zhang H.J., Chen Y.L., Liang M.J., et al., Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal. Chem. 86 (2014) 9846–9852. DOI:10.1021/ac502446m |
| [12] | Xu Y.L., Niu X.Y., Zhang H.J., et al., Switch-on fluorescence sensing of glutathione in food samples based on a graphitic carbon nitride quantum dot (g-CNQD)-Hg2+ chemosensor. J. Agric. Food Chem. 63 (2015) 1747–1755. DOI:10.1021/jf505759z |
| [13] | Wang Y., Zhang Y., Jia M.Y., et al., Functionalization of carbonaceous nanodots from MnⅡ-coordinating functional knots. Chem. Eur. J. 21 (2015) 14843–14850. DOI:10.1002/chem.201502463 |
| [14] | Xu Q., Kuang T.R., Liu Y., et al., Heteroatom-doped carbon dots:synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 4 (2016) 7204–7219. DOI:10.1039/C6TB02131J |
| [15] | Liu R.J., Zhao J.J., Huang Z.R., et al., Nitrogen and phosphorus co-doped graphene quantum dots as a nano-sensor for highly sensitive and selective imaging detection of nitrite in live cell. Sens. Actuators B 240 (2017) 604–612. DOI:10.1016/j.snb.2016.09.008 |
| [16] | Sun X.C., Brückner C., Lei Y.. One-pot and ultrafast synthesis of nitrogen and phosphorus co-doped carbon dots possessing bright dual wavelength fluorescence emission. Nanoscale 7 (2015) 17278–17282. DOI:10.1039/C5NR05549K |
| [17] | Shi B.F., Su Y.B., Zhang L.L., et al., Nitrogen and phosphorus co-doped carbon nanodots as a novel fluorescent probe for highly sensitive detection of Fe3+ in human serum and living cells. ACS Appl. Mater. Interfaces 8 (2016) 10717–10725. DOI:10.1021/acsami.6b01325 |
| [18] | Sadhanala H.K., Nanda K.K.. Boron and nitrogen co-doped carbon nanoparticles as photoluminescent probes for selective and sensitive detection of picric acid. J. Phys. Chem.C 119 (2015) 13138–13143. DOI:10.1021/acs.jpcc.5b04140 |
| [19] | Jahan S., Mansoor F., Naz S., Lei J.P., Kanwal S.. Oxidative synthesis of highly fluorescent boron/nitrogen co-doped carbon nanodots enabling detection of photosensitizer and carcinogenic dye. Anal. Chem. 85 (2013) 10232–10239. DOI:10.1021/ac401949k |
| [20] | Miao P., Han K., Tang Y.G., et al., Recent advances in carbon nanodots:synthesis, properties and biomedical applications. Nanoscale 7 (2015) 1586–1595. DOI:10.1039/C4NR05712K |
| [21] | Rong M.C., Song X.H., Zhao T.T., et al., Synthesis of highly fluorescent P, O-g-C3N4 nanodots for the label-free detection of Cu2+ and acetylcholinesterase activity. J. Mater. Chem.C 3 (2015) 10916–10924. DOI:10.1039/C5TC02584B |
| [22] | Li X.B., Niu Z.G., Chang L.L., Chen M.X., Wang E.J.. Quinoline-based colorimetric chemosensor for Cu2+:Cu2+-induced deprotonation leading to color change. Chin. Chem. Lett. 25 (2014) 80–82. DOI:10.1016/j.cclet.2013.08.002 |
| [23] | Li P., Duan X., Chen Z.Z., et al., A near-infrared fluorescent probe for detecting copper(Ⅱ) with high selectivity and sensitivity and its biological imaging applications. Chem. Commun. 47 (2011) 7755–7757. DOI:10.1039/c1cc11885d |
| [24] | Duan Y.W., Tang H.Y., Guo Y., et al., The synthesis and study of the fluorescent probe for sensing Cu2+ based on a novel coumarin Schiff-base. Chin. Chem. Lett. 25 (2014) 1082–1086. DOI:10.1016/j.cclet.2014.05.001 |
| [25] | Zhang N., Si Y.M., Sun Z.Z., et al., Rapid selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold-silver nanoclusters with silver effect-enhanced red fluorescence. Anal. Chem. 86 (2014) 11714–11721. DOI:10.1021/ac503102g |
| [26] | Jin L.H., Han C.S.. Ultrasensitive and selective fluorimetric detection of copper ions using thiosulfate-involved quantum dots. Anal. Chem. 86 (2014) 7209–7213. DOI:10.1021/ac501515f |
| [27] | Zhang L., Zhang Z.Y., Liang R.P., Li Y.H., Qiu J.D.. Boron-doped graphene quantum dots for selective glucose sensing based on the abnormal aggregation-induced photoluminescence enhancement. Anal. Chem. 86 (2014) 4423–4430. DOI:10.1021/ac500289c |
| [28] | Tang C.C., Bando Y.S., Huang Y., Zhi C.Y., Golberg D.. Synthetic routes and formation mechanisms of spherical boron nitride nanoparticles. Adv. Funct. Mater. 18 (2008) 3653–3661. DOI:10.1002/adfm.v18:22 |
| [29] | Fei H.L., Ye R.Q., Ye G.L., et al., Boron-and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 8 (2014) 10837–10843. DOI:10.1021/nn504637y |
| [30] | Han Y.Z., Tang D., Yang Y.M., et al., Non-metal single/dual doped carbon quantum dots:a general flame synthetic method and electro-catalytic properties. Nanoscale 7 (2015) 5955–5962. DOI:10.1039/C4NR07116F |
| [31] | Rong M.C., Lin L.P., Song X.H., et al., Fluorescence sensing of chromium(Ⅵ) and ascorbic acid using graphitic carbon nitride nanosheets as a fluorescent switch. Biosens. Bioelectron. 68 (2015) 210–217. DOI:10.1016/j.bios.2014.12.024 |
| [32] | Rong M.C., Lin L.P., Song X.H., et al., A label-free fluorescence sensing approach for selective and sensitive detection of 24, 6-trinitrophenol (TNP) in aqueous solution using graphitic carbon nitride nanosheets. Anal. Chem. 87 (2015) 1288–1296. DOI:10.1021/ac5039913 |
| [33] | Rong M.C., Cai Z.X., Xie L., et al., Study on the ultrahigh quantum yield of fluorescent P, O-g-C3N4 nanodots and its application in cell imaging. Chem. Eur. J. 22 (2016) 9387–9395. DOI:10.1002/chem.201601065 |
| [34] | Bourlinos A.B., Trivizas G., Karakassides M.A., et al., Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 83 (2015) 173–179. DOI:10.1016/j.carbon.2014.11.032 |
| [35] | Ganiga M., Cyriac J.. Understanding the photoluminescence mechanism of nitrogen-doped carbon dots by selective interaction with copper ions. ChemPhysChem 17 (2016) 2315–2321. DOI:10.1002/cphc.v17.15 |
 2017, Vol. 28
2017, Vol. 28