b State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Quinoxalines, which are a group of nitrogen-containing heterocyclic compounds, have been widely used in pharmaceutical chemistry [1-3] due to their biological activity of anticancer, antimicrobial, antibiotics, etc., and also have been employed as dyes, luminescent materials, semiconductors and corrosion inhibitors [4-6]. Various synthetic methodologies have been developed for the preparation of quinoxalines. Among them, the most common approach is the condensation of 1, 2-phenylenediamines with two-carbon synthons, such as 1, 2-diketone [7-16], oxalic acids [17], epoxides [18, 19], alkynes [20, 21], α-hydroxy ketones [22-25] and vicinal diols [26] (Scheme 1). Up to date, these methods suffered from certain disadvantages, i.e. using stoichiometric or excess amounts of highly reactive oxidants or expensive transition metal catalysts, tedious product isolation procedures and harsh reaction conditions. Recently, some groups have developed one two-step route to the synthesis of quinoxalines from simple ketones and 1, 2-phenylenediamines, in which the ketones are firstly oxidized to 1, 2-diketones that are further cyclized with 1, 2-phenylenediamines [27, 28]. This approach brought about an expansion on the substrates and the enhancement of the product yields. In these methods, Cu(Ⅱ) compounds were found to be the effective catalysts. For instance, Wu [27] and Wang [28] reported that CuO/I2/DMSO or Cu(OAc)2/O2/DMF systems could efficiently catalyze the formation of quinoxalines from simple ketones with 1, 2-phenylenediamines, respectively. However, these reactions were carried out in organic solvents, which may not meet the trend of green synthetic chemistry. Therefore the development of concise, efficient and environmentally benign approaches for the synthesis of quinoxalines is of general interest.
|
Download:
|
| Scheme 1. Various routes for the synthesis of quinoxalines. | |
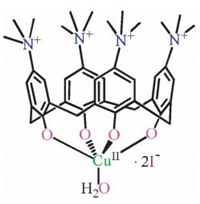
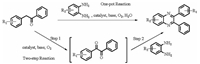
In our previous works [29-32], the water-soluble Cu(Ⅱ) complex with the zwitterionic calix[4]arene ligand L (H4L = [5, 11, 17, 23-tetrakis(trimethylammonium)-25, 26, 27, 28-tetrahydroxycalix[4]arene[Cu(Ⅱ)L(H2O)]I2 (1) (Scheme 2) exhibited excellent catalytic activity towards the oxidative polymerization of 2, 6-dimethylphenol to form poly(2, 6-dimethyl-1, 4-phenylene oxide) in water [31] and the oxidation of benzyl alcohols to benzaldehydes in water [32]. In both reactions, the zwitterionic calix[4]arene ligand L is helpful to improve the stability and solubility of compound 1 and the organic substrates in aqueous media. These results activated us to introduce catalyst 1 in the preparation of quinoxalines in water. As presented in Scheme 3, in the presence of K2CO3, one-pot aqueous-phase oxidative cyclization of deoxybenzoins with 1, 2-phenylenediamines, catalyzed by complex 1, could generate quinoxaline and its derivatives in medium to high yields, which is described below in this article.
|
Download:
|
| Scheme 2. Schematic representation of compound 1. | |
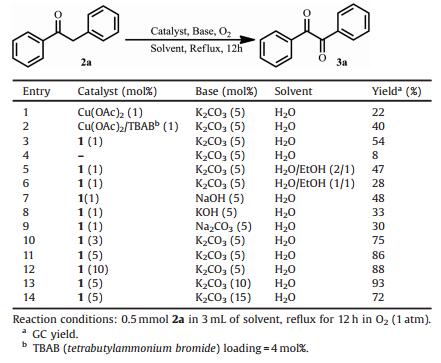
2. Results and discussion 2.1. The oxidation of deoxybenzoin catalyzed by 1
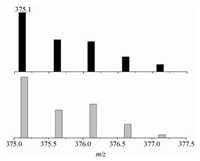
For the aforementioned two steps started from simple ketones and 1, 2-phenylenediamines, the oxidation of ketones to diketones (step 1 in Scheme 3) was more crucial and thus played the 'key step' in the plausible pathway. To this end, we firstly optimized the reaction conditions of this step. The existence of 1 in aqueous phase was studied by the positive-ion electrospray ion mass spectra (ESI-MS). One signal at m/z = 375.1 could be assigned to be the [CuL(H2O)(OH)]2+ dication (Fig. 1), implying that Cu2+ was coordinated by L in water. As listed in Table 1, the reaction of deoxybenzoin (2a, 0.5 mmol) with O2 in the presence of different copper catalysts (1.0 mol%), K2CO3 (5 mol%) in H2O (3 mL) was performed by refluxing (entries 1-4). Under the catalysis of 1, the product benzyl (3a) could be isolated in 54% yield (entry 3) after 12 h. Comparative runs without Cu(Ⅱ) salts or with Cu(OAc)2, Cu (OAc)2/TBAB under same conditions gave lower yields of benzil (8%, 22% and 40%, respectively). The reason that complex 1 showed the best catalytic activity may be attributed to the hydrophobic cavity structure of ligand L, which assisted the dissolution of the substrates in water. In entires 5 and 6, EtOH was added to the reaction system with the aim of improving the solubility of substrates. When the ratios of H2O/EtOH were gradually increased, lower yields were obtained (47% and 28%). We suppose this phenomenon was probably due to the decrease in the solubility of 1 in EtOH. The influence of bases including carbonates and hydroxides was also investigated and showed an activity order of Na2CO3 < KOH < NaOH < K2CO3 (entries 4 and 7-9). Thus subsequent studies were carried out using 1 as a catalyst along with potassium carbonate in water solution.
|
Download:
|
| Scheme 3. Two synthetic approaches to quinoxalines. | |
|
Download:
|
| Figure 1. The positive-ion ESI-MS spectrum (top) and the calculated isotope pattern (below) of the [CuL(H2O)(OH)]2+ in the aqueous phase. | |
|
|
Table 1 Optimizing the reaction conditions for the oxidation of deoxybenzoin to benzyl. |
The optimization of the catalyst and base loading was shown in Table 1 (entires 10-14). The yield of benzil gradually increased from 54% to 86% when the amount of 1 was raised from 1 mol% to 5 mol% [Cu]. It could not be evidently enhanced (88%) even if 10 mol% of 1 was added (entry 12). However, 5 mol% catalyst loading along with 10% mol K2CO3 loading could significantly increase the yield of benzil to 93% (entry 13). When more K2CO3 (15 mol%) was added, the yield dropped to 72% (entry 14). Thus, the optimal reaction conditions were identified as follows: 5 mol% catalyst 1, 10 mol% K2CO3 and 3 mL H2O as a solvent with reflux in O2 atmosphere.
2.2. The comparative synthesis of quinoxalineThe difference between the two-step reaction and the one-pot reaction was subsequently explored (Scheme 3). For the two-step approach, the mixture of deoxybenzoin, catalyst 1, K2CO3 and water was firstly refluxed for 12 h. Then 1, 2-phenylenediamine was added into the solution, which was refluxed for another 3 h to accomplish the cyclization step. The final product quinoxaline was isolated in 83% yield. In contrast, the one-pot reaction by refluxing deoxybenzoin and 1, 2-phenylenediamine under the above optimized conditions for 15 h could produce quinoxaline in a slightly higher yield (86%). Obviously, the latter one-pot procedure brought not only better substrate utilization, but also simple and convenient manipulation. However, due to the partial oxidization of 1, 2-phenylenediamine, the catalyst 1 could not be separated out and thus this catalytic system was not reusable.
2.3. The substrate scope of the one-pot oxidative cyclization for generating quinoxalinesUnder the one-pot optimal conditions, the substrate scope of the oxidative cyclization for generating quinoxalines catalyzed by 1 was examined (Table 2). The electron nature of the substituent groups on the substrates exerted different effects on the oxidation process and the cyclization process. During the oxidation process (step 1 in Scheme 3), the electron-withdrawing chloro-substituted deoxybnzoin 2b (entry 2) produced the corresponding quinoxaline 5b in a slightly higher yield (88%). While the electron-donating methoxylsubstituted deoxybenzoin 2c (entry 3) gave 5c in somewhat lower yields (52% and 78%) even after longer reaction time. The higher activity of the electron-withdrawing substituted deoxybenzoin might be attributed to the stronger acidity of the methylene group [28]. In the case of the cyclization process (step 2 in Scheme 3), 1, 2-phenylenediamines containing electron-neutral methyl-(4b-c, entries 4 and 5) and electron-donating methoxyl-(4d, entry 6) groups afforded quinoxalines in 83%-88% yields, better than those with electron-withdrawing bromo-and chloro-groups (4e-f, entries 7 and 8, yield 76%-78%). Furthermore, when stronger electron-withdrawing groups such as dichloro-, nitro-and trifluoromethyl-(4g-i, entries 9-11) were introduced on 1, 2-phenylenediamines, the yields of quinoxalines evidently decreased from 62% to 70% even after longer reaction time. However, the heterocyclo 2, 3-diaminopyridine and ortho-substituted 1, 2-phenylenediamines (4j-l, entries 12-14) remained inactive under the optimized conditions, which could be ascribed to the fact that the hetero-aromatic diamines had less reactivity in comparison with aromatic or aliphatic diamines [33, 34] and the reactivity of diamines is generally dominated by steric effects.
|
|
Table 2 Scope of the oxidative cyclization for generating quinoxalines from deoxybenzoins and 1, 2-phenylenediamines catalyzed by 1 in water. |
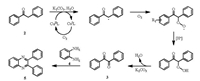
The possible mechanism of such a one-step reaction was assumed to be similar to that proposed by Wang [28] (Scheme 4). In the presence of catalyst 1, substrate 2 might firstly oxidized and deprotonated to generate a benzyl radical, which may be trapped by oxygen to produce the peroxide radical. It may yield benzyl 3 after capturing a hydrogen radical and eliminating one equivalent of H2O molecule. Then subsequent condensation and dehydratation of benzyl with 1, 2-phenylenediamine 4 give the corresponding quinoxaline 5.
|
Download:
|
| Scheme 4. Proposed mechanism for the one-pot reaction. | |
3. Conclusion
In this paper, we demonstrated a green protocol which provides a convenient route to one-pot synthesis of quinoxalines in water. Being catalyzed by the water-soluble complex 1, the oxidative cyclization of deoxybenzoins and 1, 2-phenylenediamines afforded the target quinoxaline products in good yields (up to 88%). Although their yields were somewhat lower than those of the products isolated in the organic solvents such as DMF and DMSO, it is still attractive as this work represents a useful and green protocol to introduce the water-soluble copper(Ⅱ) catalytic system for preparing quinoxalines in water. Future research will focus on improving the product yields by using other transition metal (e.g. Fe3+, Ti4+) complexes of the zwitterionic calix[4]arene ligand L.
4. Experimental 4.1. General InformationAll chemicals were purchased from commercial sources and used without purification. Water used in the catalytic studies was doubly distilled and deionized. Compound 1 was obtained according to the published procedure [31]. The oxidation products were quantitatively analyzed by GC (Agilent 7820A Gas Chromatograph with an Agilent HP-5 chromatographic column and N2 as the mobile phase) using internal standards. The 1H and 13C NMR spectra in CDCl3 were recorded at ambient temperature on a Varian UNITYplus-400 (400 MHz) spectrometer and the chemical shifts were referenced to the TMS signal. Electrospray ion mass spectra (ESI-MS) were performed on an Agilent 1200/6200 mass spectrometer using MeCN as the mobile phase.
4.2. Typical procedure for the oxidation of deoxybenzoin (2a) to benzil (3a)A Schlenk glass tube equipped with a magnetic stirring bar was charged with deoxybenzoin (2a, 0.5 mmol), complex 1 (25 mg, 0.025 mmol, 5 mol%), and K2CO3 (7 mg, 0.05 mmol, 5 mol%), which was filled with oxygen gas using an oxygen containing balloon. H2O (3.0 mL) was injected into the system via syringe under an oxygen atmosphere. After the resulting mixture was refluxed for 12 h, it was cooled to room temperature and extracted with CH2Cl2 (3 × 5 mL). The combined organic layer was washed with brine (20 mL) and dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using petroleum ether and ethyl acetate as the eluants. Compound 3a was further confirmed by its 1H and 13C NMR spectra.
4.3. Typical procedure for one-pot aqueous-phase synthesis of quinoxalines (5a-k) via oxidative cyclization of deoxybenzoins with 1, 2-phenylenediaminesA Schlenk tube equipped with a magnetic stirring bar was charged with deoxybenzoin (2a-c, 0.5 mmol), complex 1 (25 mg, 0.025 mmol, 5 mol%), and K2CO3 (7 mg, 0.05 mmol, 5 mol%), which was filled with oxygen gas using an oxygen containing balloon. H2O (3.0 mL) was added to the system via syringe under an oxygen atmosphere. After several minutes' stirring, 1, 2-phenylenediamine (4a-l, 0.75 mmol) was added to the reaction system. The mixture was further refluxed for 15 h, which was cooled to room temperature and then extracted with CH2Cl2 (3 × 5 mL). The combined organic layer was washed with brine (20 mL) and dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using petroleum ether and ethyl acetate as the eluants. All products synthesized in this work are known and confirmed by comparing their 1H and 13C NMR spectra with those found in the literatures.
AcknowledgmentsThe authors acknowledge the financial supports from the National Natural Science Foundation of China (Nos. 21271134, 21373142, 21531006 and 21671144) and the State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry (No. 2015kf-07). J. P. Lang also highly appreciates the financial supports from the "333" Project of Jiangsu Province, the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the "SooChow Scholar" Program of Soochow University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.12.035.
| [1] | Baudy R.B., Greenblatt L.P., Jirkovsky I.L., et al., Potent quinoxaline-spaced phosphono α-amino acids of the AP-6 type as competitive NMDA antagonists:synthesis and biological evaluation. J. Med. Chem. 36 (1993) 331–342. DOI:10.1021/jm00055a004 |
| [2] | Lee S.B., Park Y.I., Dong M.S., Gong Y.D.. Identification of 2, 3, 6-trisubstituted quinoxaline derivatives as a Wnt2/β-catenin pathway inhibitor in non-smallcell lung cancer cell lines. Bioorg. Med. Chem. Lett. 20 (2010) 5900–5904. DOI:10.1016/j.bmcl.2010.07.088 |
| [3] | Badran M.M., Abouzid K.A.M., Hussein M.H.M.. Synthesis of certain substituted quinoxalines as antimicrobial agents (part Ⅱ). Arch. Pharm. Res. 26 (2003) 107–113. DOI:10.1007/BF02976653 |
| [4] | Duan J.P., Sun P.P., Cheng C.H.. New Iridium complexes as highly efficient orange-red emitters in organic light-emitting diodes. Adv. Mater. 15 (2003) 224–228. DOI:10.1002/adma.200390051 |
| [5] | Schneidenbach D., Ammermann S., Debeaux M., et al., Efficient and long-time stable red Iridium(Ⅲ) complexes for organic light-emitting diodes based on quinoxaline ligands. Inorg. Chem. 49 (2010) 397–406. DOI:10.1021/ic9009898 |
| [6] | Dailey S., Feast W.J., Peace R.J., et al., Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J. Mater. Chem. 11 (2001) 2238–2243. DOI:10.1039/b104674h |
| [7] | Bhosale R.S., Sarda S.R., Ardhapure S.S., et al., An efficient protocol for the synthesis of quinoxaline derivatives at room temperature using molecular iodine as the catalyst. Tetrahedron Lett. 46 (2005) 7183–7186. DOI:10.1016/j.tetlet.2005.08.080 |
| [8] | Dhakshinamoorthy A., Kanagaraj K., Pitchumani K.. Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 52 (2011) 69–73. DOI:10.1016/j.tetlet.2010.10.146 |
| [9] | Guo W.X., Jin H.L., Chen J.X., et al., An efficient catalyst-free protocol for the synthesis of quinoxaline derivatives under ultrasound irradiation. J. Braz. Chem. Soc. 20 (2009) 1674–1679. DOI:10.1590/S0103-50532009000900016 |
| [10] | More S.V., Sastry M.N.V., Wang C.C., Yao C.F.. Molecular iodine:a powerful catalyst for the easy and efficient synthesis of quinoxalines. Tetrahedron Lett. 46 (2005) 6345–6348. DOI:10.1016/j.tetlet.2005.07.026 |
| [11] | More S.V., Sastry M.N.V., Yao C.F.. Cerium (Ⅳ) ammonium nitrate (CAN) as a catalyst in tap water:a simple, proficient and green approach for the synthesis of quinoxalines. Green Chem. 8 (2006) 91–95. DOI:10.1039/B510677J |
| [12] | Naskar S., Paira P., Paira R., et al., Montmorillonite K-10 clay catalyzed solventfree synthesis of bis-indolylindane-1, 3-dione, 2-(1', 3'-dihydro-1H-[2, 3'] biindolyl-2'-ylidene)-indan-1, 3-dione and bisindolylindeno[1, 2-b]quinoxaline under microwave irradiation. Tetrahedron 66 (2010) 5196–5203. DOI:10.1016/j.tet.2010.04.084 |
| [13] | Tingoli M., Mazzella M., Panunzi B., Tuzi A.. Elemental iodine or diphenyl diselenide in the[Bis(trifluoroacetoxy)iodo]benzene-mediated conversion of alkynes into 1, 2-diketones. Eur. J. Org. Chem. 2011 (2011) 399–404. DOI:10.1002/ejoc.v2011.2 |
| [14] | Huang T.K., Shi L., Wang R., Guo X.Z., Lu X.X.. Keggin type heteropolyacidscatalyzed synthesis of quinoxaline derivatives in water. Chin. Chem. Lett. 20 (2009) 161–164. DOI:10.1016/j.cclet.2008.10.048 |
| [15] | Khaksar S., Tajbakhsh M., Gholami M., Rostamnezhad F.. A highly efficient procedure for the synthesis of quinoxaline derivatives using polyvinylpolypyrrolidone supported triflic acid catalyst (PVPP OTf). Chin. Chem. Lett. 25 (2014) 1287–1290. DOI:10.1016/j.cclet.2014.04.008 |
| [16] | Mahesh R., Dhar A.K., Sasank TVNV T., Thirunavukkarasu S., Devadoss T.. Citric acid:an efficient and green catalyst for rapid one pot synthesis of quinoxaline derivatives at room temperature. Chin. Chem. Lett. 22 (2011) 389–392. DOI:10.1016/j.cclet.2010.11.002 |
| [17] | Ali M.M., Ismail M.M.F., El-Gaby M.S.A., Zahran M.A., Ammar Y.A.. Synthesis and antimicrobial activities of some novel quinoxalinone derivatives. Molecules 5 (2000) 864–873. DOI:10.3390/50600864 |
| [18] | Antoniotti S., Duñach E.. Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1, 2-diamines. Tetrahedron Lett. 43 (2002) 3971–3973. DOI:10.1016/S0040-4039(02)00715-3 |
| [19] | Nasar M.K., Kumar R.R., Perumal S.. Three-component tandem reactions of (2-arylsulfanyl-3-aryl-2-oxiranyl)(aryl)methanones and o-phenylenediamine:formation of quinoxalines. Tetrahedron Lett. 48 (2007) 2155–2158. DOI:10.1016/j.tetlet.2007.01.106 |
| [20] | Chandrasekhar S., Reddy N.K., Kumar V.P.. Oxidation of alkynes using PdCl2/CuCl2 in PEG as a recyclable catalytic system:one-pot synthesis of quinoxalines. Tetrahedron Lett. 51 (2010) 3623–3625. DOI:10.1016/j.tetlet.2010.05.006 |
| [21] | Wang W., Shen Y.W., Meng X., et al., Copper-catalyzed synthesis of quinoxalines with o-phenylenediamine and terminal alkyne in the presence of bases. Org. Lett. 13 (2011) 4514–4517. DOI:10.1021/ol201664x |
| [22] | Kim S.Y., Park K.H., Chung Y.K.. Manganese(Ⅳ) dioxide-catalyzed synthesis of quinoxalines under microwave irradiation. Chem. Commun. (2005) 1321–1323. |
| [23] | Marques C.S., Moura N., Burke A.J.. A simple, highly regioselective, one-pot stereoselective synthesis of tertiary α-hydroxyesters:a tandem oxidation/benzilic ester rearrangement. Tetrahedron Lett. 47 (2006) 6049–6052. DOI:10.1016/j.tetlet.2006.06.107 |
| [24] | Cho C.S., Ren W.X.. A recyclable copper catalysis in quinoxaline synthesis from α-hydroxyketones and o-phenylenediamines. J. Organomet. Chem. 694 (2009) 3215–3217. DOI:10.1016/j.jorganchem.2009.06.002 |
| [25] | Zhang C., Xu Z.J., Zhang L.R., Jiao N.. Et3N-catalyzed oxidative dehydrogenative coupling of α-unsubstituted aldehydes and ketones with aryl diamines leading to quinoxalines using molecular oxygen as oxidant. Tetrahedron 68 (2012) 5258–5262. DOI:10.1016/j.tet.2012.03.020 |
| [26] | Cho C.S., Oh S.G.. A new ruthenium-catalyzed approach for quinoxalines from o-phenylenediamines and vicinal-diols. Tetrahedron Lett. 47 (2006) 5633–5636. DOI:10.1016/j.tetlet.2006.06.038 |
| [27] | Lian M., Li Q., Zhu Y.P., Yin G.D., Wu A.X.. Logic design and synthesis of quinoxalines via the integration of iodination/oxidation/cyclization sequences from ketones and 1, 2-diamines. Tetrahedron 68 (2012) 9598–9605. DOI:10.1016/j.tet.2012.09.056 |
| [28] | Yu J.W., Mao S., Wang Y.Q.. Copper-catalyzed base-accelerated direct oxidation of C-H bond to synthesize benzils, isatins, and quinoxalines with molecular oxygen as terminal oxidant. Tetrahedron Lett. 56 (2015) 1575–1580. DOI:10.1016/j.tetlet.2015.02.019 |
| [29] | Liu L.L., Li H.X., Wan L.M., et al., A Mn(Ⅲ)-superoxo complex of a zwitterionic calix[4] arene with an unprecedented linear end-on Mn(Ⅲ)-O2 arrangement and good catalytic performance for alkene epoxidation. Chem. Commun. 47 (2011) 11146–11148. DOI:10.1039/c1cc14262c |
| [30] | Liu L.L., Ren Z.G., Wan L.M., Ding H.Y., Lang J.P.. Inclusion of unique four-clawed crown-like nitrate-water cluster[(NO3)6(H2O)6]6- anions into the inter-spaces of a 3D H-bonded cationic net formed by a cationic calix[4] arene. CrystEngComm 13 (2011) 5718–5723. DOI:10.1039/c1ce05377a |
| [31] | Wan L.M., Li H.X., Zhao W., et al., Oxidative po lymerization of 2, 6-dimethylphenol to form poly(2, 6-dimethyl-1, 4-phenylene oxide) in water through one water-soluble copper(Ⅱ) complex of a zwitterionic calix. J. Polym. Sci. Part A Polym. Chem. 50 (2012) 4864–4870. DOI:10.1002/pola.v50.23 |
| [32] | Gao J., Ren Z.G., Lang J.P.. Oxidation of benzyl alcohols to benzaldehydes in water catalyzed by a Cu(Ⅱ) complex with a zwitterionic calix. J. Organomet. Chem. 791 (2015) 88–92. |
| [33] | Bardajee G.R., Malakooti R., Jami F., Parsaei Z., Atashin H.. Covalent anchoring of copper-Schiff base complex into SBA-15 as a heterogeneous catalyst for the synthesis of pyridopyrazine and quinoxaline derivatives. Catal. Commun. 27 (2012) 49–53. DOI:10.1016/j.catcom.2012.06.028 |
| [34] | Kumar D., Seth K., Kommi D.N., Bhagat S., Chakraborti A.K.. Surfactant micelles as microreactors for the synthesis of quinoxalines in water:scope and limitations of surfactant catalysis. RSC Adv. 3 (2013) 15157–15168. DOI:10.1039/c3ra41038b |
 2017, Vol. 28
2017, Vol. 28