Pillar[n]arenes with repeating phenolic units connecting by methylene bridges at the para positions forming a pillar architecture, were pioneerly reported by Ogoshi’s group and Cao’s group [2]. As a new star macrocycle, pillar[n]arenes have attracted an explosive investigation for their versatile applications in supramolecular chemistry, nanomaterial and other fields [3-10]. Compared to traditional macrocycles such as cyclodextrin, calixarene and resorcinarene as well as calixpyrrole [11-14], pillar[n]arenes has been demonstrated that these new host macrocycles and their derivatives have ascendant host-guest properties [15-17]. Up to now, most research works on pillar[n]arenes have been focused on the five repeating units. Recently, the advanced pillar[n]arenes (n = 6-10) have also been attracted more attentions [18-20]. Copillar[5]arenes, which are containing different repeating units, were successfully prepared by co-oligomerization of different monomers [21-24]. In contrast to the fully symmetrical pillar[5]- arenes and the unsymmetrical pillar[5]arenes, copillar[5]arenes have some advantages [25-29]. Firstly, the introduction of different repeating units into pillararenes makes it possible to enhance host-guest binding. For instance, Chen and Cao [30] reported a copillar[5]arenes bearing a phosphoryl group and found it exhibiting stronger binding affinity toward guests such as 1-butanol and 1, 4-butanediol in chloroform due to multiple noncovalent interactions. Secondly, they can bear many functionalized groups, which can be formed cyclic dimers or supramolecular polymers that have potential applications in switching devices, molecular machines and formation of gels. For example, Zhang and Huang [31] reported two new copillar[5]arenes arranged in different motifs to form cyclic dimer and linear supramolecular polymers in the solid state. Thus the research and appliactions on copillar[5]arenes have obtained considerable attention [32-37]. In this paper, we wish to describe the synthesis, characterization, and metal identification of difunctionalized copillar[5]arene Schiff bases. The obtained pillar[5]arene Schiff bases were characterized by 1H NMR, 13C NMR, IR, mass spectroscopic. Besides, the molecular structures of Schiff bases were determined by single crystal X-ray diffraction, which unambiguously confirmed 3D columnar structure. Meanwhile, the coordination behavior of the obtained copillar[5]arene Schiff bases as polydentated ligands were investigated by UV-vis and fluorescence spectroscopy.
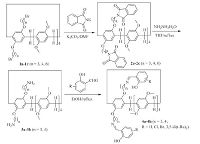
2. Results and discussionThe synthetic methods for the difunctionalized copillar[5]arene Schiff bases were illustrated in Scheme 1. The dibromo-functionalized copillar[5]arenes 1a-1c (n = 3, 4, 6) were prepared in about 20% yields according to the previously reported method [38, 39], it provides a good way to further introducing functionalized groups on the copillar[5]arene. The diphthalimido copillar[5]arenes 2a-2c (n = 3, 4, 6) were easily prepared by alkylation with potassium phthalimide in DMF in nearly very high yields. Then, the desired diamino-functionalized copillar[5]arenes 3a-3b (n = 3, 4) was successfully prepared by standard Gabriel reaction. However, the yield of the corresponding copillar[5]arene with di (aminohexyl) groups 3c was very low, which was not employed in the further reaction. The condensation reaction of diamino-functionalized pillar[5]arenes 3a-3b (n = 3, 4) with salicylaldehyde and its 5- chloro, 5-bromo, 3, 5-di (t-butyl) substituted derivatives in ethanol resulted in the corresponding difunctionalized copillar[5]arene Schiff bases 4a-4h in good yields. The structures of the prepared functionalized copillar[5]arenes were fully characterized by IR, HRMS, 1H and 13C NMR spectra.
|
Download:
|
| 1. Synthesis of the difunctionalized copillar[5]arene Schiff bases. | |
The 1H NMR spectra of diamino-functionalized copillar[5]arenes 3a showed two triplets at δ 3.86, 2.72 and one mixed peaks at 1.78-1.75 ppm for the two propylene groups. The absorption of the bridged methylene units overlapped with the peaks of methyoxy groups. But the absorptions for active amino groups were not observed in the spectra. In the 1H NMR spectra of Schiff base 4b, the hydroxy group displays a broad peak at 13.32 ppm and the proton of aldimine unit displays a singlet at 8.27 ppm. The methoxy groups showed three peaks at δ 3.63, 3.56 and 3.53, respectively. The two propylenes showed two peaks at 3.88 and 2.05 ppm. The absorption signs of two propylene group at normal magnetic fields indicated that the two chains of Schiff base units exist outside of the cavity of pillar[5]arene, which is different to that of the similar copillar[5]arene mono-Schiff bases [37].
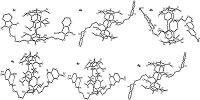
The single crystal structures of difunctionalized pillar[5]arene Schiff bases 2c, 4a, 4b, 4c, 4e and 4g were successfully determined by X-ray diffraction method. The structural figures were drawn in Fig. 1. The single crystal structures confirmed the unique symmetrical pillar architecture that is composed of phenolic units connecting by methylene bridges at the para-position. As shown in Fig. 1, the two phthalimidohexyl groups stretched to the opposite direction of pillararene core and the whole molecule is in symmetric pattern. The five difunctionalized pillar[5]arene Schiff bases have similar structural pattern, in which the length of alkyl chain and the substituent at aryl group showed neglected effect on the crystal structure. The two Schiff base moieties located above and below the cavity of pillararenes, respectively. The Schiff base moiety did not penetrated into the cavity of pillararene to form rotaxane. Two intramolecular hydrogen bonds were also observed between the phenolic hydroxy groups and imine groups.
|
Download:
|
| Figure 1. Single crystal structures of pillar[5]arene Schiff bases 2c, 4a, 4b, 4c, 4e and 4g. | |
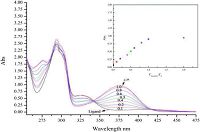
n order to examine the complexing ability of the obtained difunctionalized pillar[5]arene Schiff bases, the coordination properties of one representative compound 4b to some transition metal ions were investigated by UV-vis and fluorescence spectroscopy. The common UV-vis spectral feature of the compound showed two intense absorption bands in the range of 285 nm and 325 nm. The band at 285 nm is described as π-π* transition and second band at 325 nm could be assigned to transition of n-π* in Schiff base moiety. The changes in the absorption of 4b was investigated in dichloromethane-methanol (v/v = 1:1) at room temperature after addition of various metal ions Cu2+, Co2+, Zn2+, Ni2+, Mn2+, Pd2+ and Cd2+. As shown in Fig. 2, when transition metal Zn2+, Cu2+, Ni2+, Co2+ were introduced, a new absorption peak appeared at region of 325 nm, which means these transition metal ions showing stronger complexation to Schiff base ligand 4b. Notably, the strongest absorption peak at about 380 nm was observed comparing with its original absorption peak 325 nm in case of Zn2+ ion. Therefore, the titration of Schiff base 4b with different concentration of Zn2+ were determined in UV spectra. As seen in Fig. 3, the molar ratios of ligands 4b to Zn2+ were determined as 1:1, which also indicat
|
Download:
|
| Figure 3. UV-vis spectra of ligands 4b (1 × 10-4 mol/L) with different ratio of Zn2+/4b: 0, 0.1, 0.2, 0.4, 0.5, 0.6, 0.8, 1.0, 2.0. | |
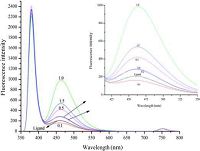
The fluorescence spectroscopy of Schiff base 4b with zinc ions were also measured (Fig. 4). With the increase of the concentration of zinc ion, the fluorescence enhanced gradually and reached to the highest until the concentration of zinc ion at 1.0 equivalent. Then, the fluorescence will decrease with the increase of concentration of zinc ion. This result also indicated that a 4b-Zn metal complex with 1:1 stoichiometry was formed in the solution. Both UV-vis spectra and fluorescence spectra indicates metal complex 4b-Zn has a 1:1 stoichiometry. Because the two coordinate Schiff base moieties located on the above and below the cavity of pillar[5]arene in ligand 4b, the coordination mode for the complex 4b-Zn might be forming a cyclic complex with two metal ions and two ligands or forming a 1D coordination polymer. Due to lack of single crystal structure of the metal complex, it is difficult to elucidate the actual coordination mode.
|
Download:
|
| Figure 4. UV-vis spectra of ligands 4b (1 × 10-6 mol/L) with different ratio of Zn2+/4b: 0, 0.1, 0.2, 0.5, 1.0, 1.5, 2.0. | |
3. Conclusion
In conclusion, a series of difunctionalized copillar[5]arene Schiff bases was designed and synthesized, the synthesis is straightforward and the yields are usually satisfactory. Six single crystal structures of the copillar[5]arene derivatives were successfully determined by X-ray diffraction method. As a necessary supplement to the pillararene family, difunctionalized pillar[5]arene Schiff bases showed good binding properties with transition metal. These investigations open up a way for the studies of ion recognition about pillararenes. The recognition abilities toward different cations (Cu2+, Co2+, Zn2+, Ni2+, Mn2+, Pd2+, Cd2+) has been investigated by UV spectra. Future works will focus on the syntheses of new functional pillararene derivatives, and their applications in the construction of effective ion recognition motifs.
4. Experimental 4.1. Chemicals and apparatusAll reagents were commercially available and used as supplied without further purification. The dibromo-functionalized pillar[5]arene was prepared according to the published procedure [38, 39]. 1H NMR and 13C spectra were collected on either a Agilent DD2 400 MHz spectrometer or a Bruker AV-600 MHz spectrometer with internal standard tetramethylsilane (TMS) and signals as internal references, and the chemical shifts (δ) were expressed in ppm. High-resolution mass (ESI) spectra were obtained with Bruker Micro-TOF spectrometer. The Fourier transform infrared (FTIR) samples were prepared as thin films on KBr plates, and spectra were recorded on a Bruker Tensor 27 spectrometer and are reported in terms of frequency of absorption (cm-1). The single crystals were determined on a Bruker Smart APEX-2 diffractometer.
4.2. Synthesis of diphthalimido-functionalized copillar[5]arenes (2a, 2b)A solution of dibromo-functionalized copillar[5]arene 1a-1c (2.0 mmol) and potassium phthalimide (4.5 mmol) were heated in DMF (40.0 mL) at 50 ℃ overnight. After adding water (100 mL), the resulting yellow precipitates were collected by filtration and dried to give crude product. The crude product was purified by column chromatography on silica gel with petroleum ether/ethyl acetate = 2/1 as the eluent to give the product.
4.3. Synthesis of diamino-functionalized copillar[5]arenes (3a-3b)The above obtained products 2a-2b (2.0 mmol) was dissolved in THF (150 mL). Hydrazine monohydrate (85%, 2.0 mL) was added. The mixture was refluxed for 24 h. After removing the solvent at reduced pressure, the residue was subjected to column chromatography on silica gel with dichloromethane/methanol = 3/1 as the eluent to give the pure di-amino-functionalized pillar[5]arenes.
Diamino-functionalized copillar[5]arene (3a, n = 3) : White solid, 87%, m.p. 106-108 ℃; 1H NMR (400 MHz, DMSO-d6) : δ 6.75 (d, 10H, J = 4.2 Hz, ArH), 3.86 (t, 4H, J = 6.1 Hz, CH2), 3.64-3.63 (m, 34H, CH2, OCH3), 2.72 (t, 4H, J = 6.3 Hz, CH2), 1.78-1.75 (m, 4H, CH2); 13C NMR (100 MHz, DMSO-d6) : δ 150.4, 150.3, 149.6, 128.1, 128.0, 128.0, 127.9, 114.6, 113.8, 113.8, 113.7, 113.6, 113.6, 113.6, 113.5, 66.1, 55.9, 55.9, 55.8, 38.8, 33.0, 33.0, 30.9, 29.7, 29.5, 29.4, 29.3; IR (KBr, cm-1) : y 3364, 2940, 2836, 1712, 1656, 1503, 1461, 1399, 1309, 1212, 1095, 1043, 931, 874, 774, 713, 651; MS (m/z) : HRMS (ESI) calcd. for C49H61N2O10 ([M+H]+) : 837.4321; found: 837.4319.
Diamino-functionalized copillar[5]arene (3b, n = 4) : White solid, 85%, m.p. 108-110 ℃; 1H NMR (400 MHz, DMSO-d6) : d 6.78 (s, 10H, ArH), 3.82 (t, 4H, J = 6.0 Hz, CH2), 3.67-3.65 (m, 34H, CH2, OCH3), 2.60 (t, 4H, J = 6.8 Hz, CH2), 1.77-1.72 (m, 4H, CH2), 1.57-1.52 (m, 4H, CH2); 13C NMR (100 MHz, DMSO-d6) : δ 150.4, 150.4, 149.6, 128.1, 128.0, 128.0, 127.9, 114.6, 113.8, 113.8, 113.7, 113.7, 68.1, 55.9, 55.9, 41.1, 30.9, 29.4, 29.4, 29.4, 29.3, 28.9, 27.1; IR (KBr, cm-1) : y 3431, 2937, 2839, 1614, 1503, 1461, 1399, 1309, 1212, 1096, 1043, 931, 874, 712, 651; MS (m/z) : HRMS (ESI) calcd. for C51H65N2O10 ([M+H]+) : 865.4634; found: 865.4640.
4.4. Synthesis of difunctionalized copillar[5]arene Schiff bases (4a-4h)A suspension of diamino-functionalized copillar[5]arenes 3a-3b (0.20 mmol) and salicylaldehyde or its derivatives (0.50 mmol) in ethanol (20.0 mL) was refluxed for 12 h. After cooling, the resulting precipitate was collected by filtration and washed with cold alcohol to give the yellow solid.
Difunctionalized pillar[5]arene Schiff base (4a: n = 3, R = H) : Yellow solid, 77%, m.p. 134-136 ℃; 1H NMR (400 MHz, CDCl3) : δ 13.29 (brs, 2H, OH), 8.34 (s, 2H, CH=N), 7.33 (t, 2H, J = 8.0 Hz, ArH), 7.24 (s, 1H, ArH), 6.99 (brs, 2H, ArH), 6.87 (t, 2H, J = 7.5 Hz, ArH), 6.79 (s, 2H, ArH), 6.72-6.64 (m, 9H, ArH), 3.94-3.88 (m, 8H, CH2), 3.77 (d, 10H, J = 8.2 Hz, CH2), 3.65-3.63.61 (m, 15H, OCH3), 3.58-3.57 (m, 6H, OCH3), 3.52 (s, 3H, OCH3), 2.06 (brs, 4H, CH2); 13C NMR (100 MHz, CDCl3) : δ 165.5, 161.2, 150.8, 150.7, 149.8, 132.2, 131.3, 128.4, 128.3, 128.2, 128.1, 118.7, 118.6, 116.9, 114.9, 114.2, 114.0, 113.9, 65.6, 56.3, 55.9, 55.8, 55.7, 30.9, 29.7, 29.7, 29.6, 29.6; IR (KBr, cm-1) : υ 3431, 3053, 2940, 2836, 1630, 1502, 1461, 1401, 1279, 1211, 1045, 988, 931, 876, 764, 652; MS (m/z) : HRMS (ESI) calcd. for C63H69N2O12 ([M+H]+) : 1045.4845; Found: 1045.4843.
Difunctionalized copillar[5]arene Schiff base (4b: n = 3, R = Cl) : Yellow solid, 74%, m.p. 138-140 ℃; 1H NMR (400 MHz, CDCl3) : δ 13.32 (brs, 2H, OH), 8.27 (s, 2H, CH=N), 7.22 (s, 2H, ArH), 6.92 (d, 2H, J = 8.4 Hz, ArH), 6.78 (s, 2H, ArH), 6.72-6.66 (m, 10H, ArH), 3.88 (t, 8H, J = 5.6 Hz, CH2), 3.77 (d, 10H, J = 7.4 Hz, CH2), 3.63 (s, 12H, OCH3), 3.56 (s, 6H, OCH3), 3.53 (s, 6H, OCH3), 2.05 (brs, 4H, CH2); 13C NMR (100 MHz, CDCl3) : δ 164.4, 159.8, 150.8, 150.7, 149.8, 132.1, 130.4, 128.5, 128.5, 128.2, 128.1, 123.1, 119.4, 118.5, 114.9, 114.2, 114.1, 113.8, 65.4, 56.2, 55.9, 55.9, 55.8, 55.7, 30.7, 29.7, 29.6; IR (KBr, cm-1) : υ 3470, 3057, 2942, 2834, 1631, 1496, 1467, 1399, 1274, 1211, 1046, 987, 876, 821, 773, 700, 649; MS (m/z) : HRMS (ESI) calcd. for C63H67Cl2N2O12 ([M+H]+) : 1113.4066; Found: 1113.4050.
Difunctionalized copillar[5]arene Schiff base (4g: n = 4, R = Br) : Yellow solid, 75%, m.p. 116-118 ℃; 1H NMR (400 MHz, CDCl3) : δ 13.51 (brs, 2H, OH), 8.12 (s, 2H, CH=N), 7.39-7.36 (m, 2H, ArH), 7.33 (d, 2H, J = 1.9 Hz, ArH), 6.85 (d, 2H, J = 8.7 Hz, ArH), 6.78-6.69 (m, 10H, ArH), 3.88-3.85 (m, 4H, CH2), 3.78-3.73 (m, 10H, CH2), 3.65 (s, 12H, OCH3), 3.59 (s, 6H, OCH3), 3.55 (s, 6H, OCH3), 3.48 (brs, 4H, CH2), 1.76 (brs, 8H, CH2); 13C NMR (100 MHz, CDCl3) : δ 163.7, 160.6, 150.8, 150.8, 150.8, 150.7, 150.0, 134.8, 133.3, 128.4, 128.4, 128.2, 128.2, 120.0, 119.1, 115.1, 114.2, 114.2, 114.1, 113.9, 109.8, 67.9, 58.8, 55.9, 55.8, 55.8, 29.7, 27.5, 27.5; IR (KBr, cm-1) : υ 3585, 3070, 2940, 2836, 1632, 1497, 1397, 1276, 1210, 1043, 987, 936, 876, 822, 774, 691; MS (m/z) : HRMS (ESI) calcd. for C65H71Br2N2O12 ([M+H]+) : 1229.3368; found: 1229.3381.
Difunctionalized copillar[5]arene Schiff base (4h: n = 4, R = 3, 5- di (t-Bu) ): Yellow solid, 71%, m.p. 98-100 ℃; 1H NMR (400 MHz, CDCl3) : δ 13.81 (brs, 2H, OH), 8.36 (s, 2H, CH=N), 7.38 (d, 2H, J = 2.3 Hz, ArH), 7.08 (d, 2H, J = 2.3 Hz, ArH), 6.79-6.74 (m, 10H, ArH), 3.87-3.84 (m, 8H, CH2), 3.77-3.72 (m, 10H, CH2), 3.65-3.62 (m, 21H, OCH3), 3.57 (s, 3H, OCH3), 1.88 (brs, 8H, CH2), 1.45 (s, 18H, CH3), 1.31 (s, 18H, CH3); 13C NMR (100 MHz, CDCl3) : δ 166.0, 158.1, 150.8, 150.8, 150.7, 150.7, 150.0, 140.0, 136.7, 128.4, 128.3, 128.3, 128.2, 128.1, 126.8, 125.8, 117.8, 115.0, 114.1, 114.0, 113.9, 68.1, 59.3, 55.8, 55.8, 55.7, 55.7, 35.0, 34.1, 31.5, 29.7, 29.6, 29.6, 29.4, 27.9, 27.5; IR (KBr, cm-1) : υ 3410, 3052, 2950, 2865, 1630, 1502, 1464, 1399, 1314, 1211, 1045, 990, 930, 874, 774, 715, 652; MS (m/z) : HRMS (ESI) calcd. for C81H105N2O12 ([M+H]+) : 1297.7662; found: 1297.7664.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21172190, 21372192) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank the Analysis and Test Center of Yangzhou University providing instruments for analysis.
| [1] | T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi, Y. Nakamoto, para-Bridged symmetrical pillar[5]arenes:their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 130 (2008) 5022–5023. DOI:10.1021/ja711260m |
| [2] | D.R. Cao, Y.H. Kou, J.Q. Liang, A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 48 (2009) 9721–9723. DOI:10.1002/anie.200904765 |
| [3] | M. Xue, Y. Yang, X.D. Chi, Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 45 (2012) 1294–1308. DOI:10.1021/ar2003418 |
| [4] | P.J. Cragg, K. Sharma, Pillar[5]arenes:fascinating cyclophanes with a bright future. Chem. Soc. Rev. 41 (2012) 597–607. DOI:10.1039/C1CS15164A |
| [5] | T. Ogoshi, K. Kitajima, T. Aoki, Synthesis and conformational characteristics of alkyl-substituted pillar[5]arenes. J. Org. Chem. 75 (2010) 3268–3273. DOI:10.1021/jo100273n |
| [6] | T. Ogoshi, T. Aoki, K. Kitajima, Facile, rapid, and high-yield synthesis of pillar[5]arene from commercially available reagents and its X-ray crystal structure. J. Org. Chem. 76 (2011) 328–331. DOI:10.1021/jo1020823 |
| [7] | T. Ogoshi, D. Yamafuji, D. Kotera, Clickable di- and tetrafunctionalized pillar[n]arenes (n=5, 6) by oxidation-reduction of pillar[n]arene units. J. Org. Chem. 77 (2012) 11146–11152. DOI:10.1021/jo302283n |
| [8] | Y. Chen, H.Q. Tao, Y.H. Kou, Synthesis of pillar[7] arene. Chin. Chem. Lett. 23 (2012) 509–511. DOI:10.1016/j.cclet.2012.03.026 |
| [9] | T. Ogoshi, K. Demachi, K. Masaki, Synthesis of meso-pillar[6] arenes by bridging between hydroquinone units in an alternating up-and-down manner. Chem. Commun. 49 (2013) 3952–3954. DOI:10.1039/c3cc41592a |
| [10] | T. Ogoshi, N. Ueshima, T. Akutsu, The template effect of solvents on high yield synthesis, co-cyclization of pillar[6] arenes and interconversion between pillar[5]-and pillar[6] arenes. Chem. Commun. 50 (2014) 5774–5777. DOI:10.1039/c4cc01968g |
| [11] | J.J. Sun, Y. Han, J. Sun, C.G. Yan, Synthesis and crystal structure of Ni, Cu complexes of 5-methyl-10,10,15,15,20,20-hexaethylcalix[4] pyrrole mono-Schiff bases. Chin. Chem. Lett. 26 (2015) 685–689. DOI:10.1016/j.cclet.2015.03.028 |
| [12] | W.W. Gu, W.J. Chen, C.G. Yan, Synthesis and crystal structures of Ag and Hg complexes of bis(N-heterocyclic carbenes) on p-tert-butylcalix[4] arene plateform. Supramol. Chem. 27 (2015) 407–413. DOI:10.1080/10610278.2014.965709 |
| [13] | L. Li, J. Sun, L.L. Zhang, Y. Yao, C.G. Yan, Crystal structure and fluorescence sensing properties of tetramethoxyresorcinarene functionalized Schiff bases. J. Mole. Struct. 1081 (2015) 355–436. DOI:10.1016/j.molstruc.2014.10.064 |
| [14] | L. Li, Y. Yao, J. Sun, C.G. Yan, Preparation and application of tubular assemblies based on amphiphilic tetramethoxyresorcinarenes. RSC Adv. 5 (2015) 102454–102461. DOI:10.1039/C5RA22289C |
| [15] | W.B. Hu, C.D. Xie, W.J. Hu, Selectivity and cooperativity in the binding of multiple guests to a pillar[5]arene-crown ether fused tricyclic host. J. Org. Chem. 80 (2015) 7994–8000. DOI:10.1021/acs.joc.5b01038 |
| [16] | G.C. Yu, B. Hua, C.Y. Han, Proton transfer in host-guest complexation between a difunctional pillar[5]arene and alkyldiamines. Org. Lett. 16 (2014) 486–2489. |
| [17] | X.B. Hu, Z.X. Chen, G. Tang, J.L. Hou, Z.T. Li, Single-molecular artificial transmembrane water channels. J. Am. Chem. Soc. 134 (2012) 8384–8387. DOI:10.1021/ja302292c |
| [18] | X.B. Hu, Z. Chen, L. Chen, Pillar[n]arenes (n=8-10) with two cavities:synthesis, structures and complexing properties. Chem. Commun. 48 (2012) 10999–11001. DOI:10.1039/c2cc36027f |
| [19] | Z.T. Li, J. Yang, G.C. Yu, Synthesis of a water-soluble pillar[9] arene and its pH-responsive binding to paraquat. Chem. Commun. 50 (2014) 2841–2843. DOI:10.1039/c3cc49535c |
| [20] | J. Zhou, M. Chen, G.W. Diao, Synthesis of the first amphiphilic pillar[6] arene and its enzyme-responsive self-assembly in water. Chem. Commun. 50 (2014) 11954–11956. DOI:10.1039/C4CC05621C |
| [21] | Z.B. Zhang, B.Y. Xia, C.Y. Han, Y.H. Yu, F.H. Huang, Syntheses of copillar[5]-arenes by co-oligomerization of different monomers. Org. Lett. 12 (2010) 3285–3287. DOI:10.1021/ol100883k |
| [22] | Y.M. Zhang, B.B. Shi, H. Li, Copillar[5]arene-based supramolecular polymer gels. Polym. Chem. 5 (2014) 4722–4725. DOI:10.1039/C4PY00186A |
| [23] | L.Z. Liu, L.Y. Wang, C.C. Liu, Dimerization control in the self-assembly behavior of copillar[5]arenes bearing v-hydroxyalkoxy groups. J. Org. Chem. 77 (2012) 9413–9417. DOI:10.1021/jo301779y |
| [24] | T. Ogoshi, K. Demachi, K. Kitajima, T. Yamagishi, Monofunctionalized pillar[5]-arenes:synthesis and supramolecular structure. Chem. Commun. 47 (2011) 7164–7166. DOI:10.1039/c1cc12333e |
| [25] | N.L. Strutt, R.S. Forgan, J.M. Spruell, Y.Y. Botros, J.F. Stoddart, Monofunctionalized pillar[5]arene as a host for alkanediamines. J. Am. Chem. Soc. 133 (2011) 5668–5671. DOI:10.1021/ja111418j |
| [26] | M.F. Ni, Y.F. Guan, L. Wu, Improved recognition of alkylammonium salts by ion pair recognition based on a novel heteroditopic pillar[5]arene receptor. Tetrahedron Lett. 53 (2012) 6409–6413. DOI:10.1016/j.tetlet.2012.09.043 |
| [27] | L.Y. Gao, S.Y. Dong, B. Zheng, F.H. Huang, Synthesis of a pillar[5]arene-based heteroditopic host and its complexation with n-octyltriethylammonium salts. Eur. J. Org. Chem. 7 (2013) 1209–1213. |
| [28] | M.F. Ni, X.Y. Hu, J.L. Jiang, L.Y. Wang, The self-complexation of mono-ureafunctionalized pillar[5]arenes with abnormal urea behaviors. Chem. Commun. 50 (2014) 1317–1319. DOI:10.1039/C3CC47823H |
| [29] | S. Sun, J.B. Shi, Y.P. Dong, A pillar[5]arene-based side-chain pseudorotaxanes and polypseudorotaxanes as novel fluorescent sensors for the selective detection of halogen ions. Chin. Chem. Lett. 24 (2013) 987–992. DOI:10.1016/j.cclet.2013.07.014 |
| [30] | Y. Chen, M.Q. He, B.Q. Li, A monophosphoryl copillar[5]arene:synthesis and host-guest complexation with alkanols. RSC Adv. 3 (2013) 21405–21408. DOI:10.1039/c3ra43744b |
| [31] | Z.B. Zhang, G.C. Yu, C.Y. Han, Formation of a cyclic dimer containing two mirror image monomers in the solid state controlled by van der waals forces. Org. Lett. 18 (2011) 4819–4821. |
| [32] | C.L. Sun, J.F. Xu, Y.Z. Chen, Monofunctionalized pillar[5]arene-based stable[1] pseudorotaxane. Chin. Chem. Lett. 26 (2015) 843–846. DOI:10.1016/j.cclet.2015.05.030 |
| [33] | Y.L. Wang, G.C. Ping, C.J. Li, Efficient complexation between pillar[5]arenes and neutral guests:from host-guest chemistry to functional materials. Chem. Commun. 52 (2016) 9858–9872. DOI:10.1039/C6CC03999E |
| [34] | H.Q. Chen, J.Z. Fan, X.S. Hu, Biphen[n]arenes. Chem. Sci. 6 (2015) 197–202. DOI:10.1039/C4SC02422B |
| [35] | S.L. Wang, Y.L. Wang, Z.X. Chen, The marriage of endo-cavity and exo-wall complexation provides a facile strategy for supramolecular polymerization. Chem. Commun. 51 (2015) 3434–3437. DOI:10.1039/C4CC08820D |
| [36] | C.J. Li, Pillararene-based supramolecular polymers:from molecular recognition to polymeric aggregates. Chem. Commun. 50 (2014) 12420–12433. DOI:10.1039/C4CC03170A |
| [37] | Y. Han, G.F. Huo, J. Sun, Formation of a series of stable pillar[5]arene-based pseudo[1]-rotaxanes and their[1] rotaxanes in the crystal state. Sci. Rep. 6 (2016) 28748. DOI:10.1038/srep28748 |
| [38] | N.L. Strutt, D. Fairen-Jimenez, J. Iehl, Incorporation of an A1/A2-difunctionalized Pillar[5]arene into a metal-organic framework. J. Am. Chem. Soc. 134 (2012) 17436–17439. DOI:10.1021/ja3082523 |
| [39] | K. Wang, L.L. Tan, D.X. Chen, One-pot synthesis of pillar[n]arenes catalyzed by a minimum amount of TfOH and a solution-phase mechanistic study. Org. Biomol. Chem. 10 (2012) 9405–9409. DOI:10.1039/c2ob26635k |
 2017, Vol. 28
2017, Vol. 28 


