b Tianjin Key Laboratory of Materials Laminating Fabrication and Interface Control Technology, Hebei University of Technology, Tianjin 300130, China
Due to their high power density and energy-conversion efficiency, direct methanol fuel cells (DMFCs) are promising candidates for portable, transportation and mobile applications [1-4]. Pt nanoparticles supported on carbon materials have been recognized among the most active electrocatalysts for methanol oxidation [5-7]. However, the use of carbon material supported Pt catalyst usually leads to a thicker catalyst layer in the membrane- electrode assembly (MEA) of the DMFC compared with the use of the same mass of unsupported Pt catalyst, resulting in the increased inner electrical resistance and diffusion length in the catalyst layer. Due to this issue, the design of catalysts with higher loading of Pt nanoparticles on the carbon supports may be a promising strategy for the fabrication of thin-layered MEA.
Since high Pt loading can unavoidably lead to the increased size and agglomeration of Pt particles, great efforts have been focused on developing various carbon materials with particular structures to improve the distribution and restrict the growth of the Pt nanoparticles. In this regard, a variety of nanostructured carbon materials, such as graphene [8, 9], carbon black [10-12], carbon nanotubes [13-16], and mesoporous carbons [17-19], have been applied to achieve a high Pt loading (>50 wt%) catalyst while maintaining a high dispersion of small nanoparticles. Among them, orderedmesoporous carbon materials [17, 20] have been considered as promising high loading catalyst supports, because they provide a high surface area for highly dispersed Pt nanoparticles and ordered uniform mesopores for ion diffusion. In our previous study, a hierarchical nanostructured carbon (OMCS)with a mesopore size of ca. 8 nm had been used as a catalyst support to disperse Pt with low Pt loading (20 wt%) [21]. The preliminary results showed that the OMCS-supported Pt (20 wt%) catalyst has significantly enhanced electrocatalytic performance compared with the carbon blacksupported Pt. Inspired by the results, hereinwe employ theOMCS to support Pt nanoparticles with high Pt loading (60 wt%). The higher loading of Pt nanoparticles supported onOMCS is very important for reducing the diffusion layer thicknessandmass transport resistance. In addition, the ordered bicontinuous macropores (between the close-packed mesoporous carbon spheres) facilitate the mass transport of fluids, allowing for enhanced access of reactant molecules to the active sites, while the mesopores provide the large surface area for high dispersion of Pt nanoparticles. In this work, we first demonstrate the preparation of high loading (60 wt%) Pt nanoparticles supported on OMCS and investigate their electrocatalytic properties for the methanol oxidation reaction (MOR).
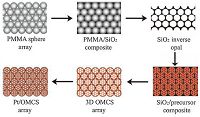
2. Experimental 2.1. Synthesis of the Pt/OMCS catalystScheme 1 illustrates the procedure for the fabrication of the Pt/OMCS. The detailed preparation is described below.
|
Download:
|
| Scheme. 1. Schematic for the formation process of the Pt/OMCS. | |
Synthesis of 3D ordered mesoporous carbon sphere arrays (OMCS): Monodisperse PMMA microspheres with ca. 460 nm in diameter were synthesized using a previously reported method [22]. The resulting PMMA microsphere suspension was then transferred to a glass bottle and allowed to stand for several weeks at room temperature to allow the spheres to precipitate completely. After the water was evaporated, PMMA colloidal crystal monoliths were formed. The prepared PMMA colloidal crystal monoliths were heated at 120 ℃ for 15 min to induce a stronger contact between each of the spheres. Then, the PMMA colloidal crystal monolith was immersed in the silica precursor solution (TEOS:0.1 mol L-1 HCl:ethanol = 1:1:1.5 in weight ratio) for 1 h and then removed carefully from the solution and dried in air at room temperature for 24 h. The freestanding silica inverse opal was obtained after removal of the PMMA spheres by heating at 450 ℃ for 5 h. For the formation of the ordered mesoporous carbon sphere arrays, 1.0 g silica inverse opal was immersed in 4.0 g of a homogeneous ethanol solution containing 1.0 g of resol (Mw < 500) and 0.5 g of amphiphilic triblock copolymer Pluronic F127 for 1 h. The impregnated composites were maintained at 25 ℃ for 6 h to evaporate the ethanol, followed by heating at 100 ℃ for 24 h. The resulting composites were then heated in N2 at 350 ℃ for 2 h at a heating rate of 1 ℃ min-1 to remove the F127, and at 5 ℃ min-1 rising to 900 ℃, followed by a 2 h soak for further carbonation [23, 24]. Finally, the OMCS was obtained by etching the silica inverse opal with a 5 wt% HF solution for 48 h.
Deposition of Pt on OMCS: ~0.05 g of the OMCS was dispersed in a 2 mL solution of 0.2 g of H2PtCl6.6H2O in ethanol and ultrasonicated for 20 min. After drying at room temperature, the resulting powder was placed in a ceramic boat and reduced at 150 ℃ for 2 h in a H2/Ar flow.
2.2. CharacterizationScanning electron microscopy (SEM) images were obtained on a Hitachi S-4700 FEG scanning electron microscope. Transmission electron microscopy (TEM) and high-resolution TEM (HR-TEM) were carried out on a Hitachi H800 and a JEOL JEM-3010 transmission electron microscope, respectively (both operating at 200 kV). Powder X-ray diffraction (XRD) data were collected on a Shimadzu XRD-6000 diffractometer with Cu Ka radiation (λ = 1.5418 Å). The actual Pt contents of the catalysts were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES, IRIS Intrepid Ⅱ XSP, Thermo Elemental).
2.3. Electrochemical experimentsThe electrochemical measurements were performed in a potentiostat/galvanostat (Reference 600, Gamry Instruments) using a conventional three-electrode cell with a double junction Ag/AgCl (saturated KCl) electrode and a Pt wire as the reference and counter electrodes, respectively. To prepare the working electrode, 1 mg of the catalyst was ultrasonically dispersed in 1 mL of ethanol. Subsequently, 5 mL of the suspension was deposited onto a 4 mm glassy carbon (GC) electrode. After the electrode was dried at room temperature, 5 mL of a Nafion solution (5 wt%) was placed on the top of the GC substrate and dried to completion at room temperature. The total Pt loadings were controlled at ~0.02 mg cm-2 . The cyclic voltammetry (CV) tests were performed in an Ar-saturated 0.5 mol L-1 H2SO4 solution either with or without 1 mol L-1 CH3OH at room temperature at a scan rate of 50 mV s-1. All the potentials were converted to the normal hydrogen electrode (NHE) scale using the equation ENHE = EAg/AgCl;sat. KCl + 0.197 V.
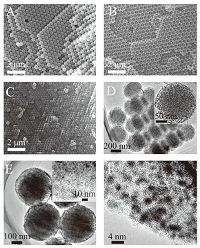
3. Results and discussionFig. 1A shows a typical SEM image of the 3D ordered PMMA sphere arrays (PMMA opal) prepared from highly monodisperse PMMA spheres (ca. 460 nm). The SEM image of the silica inverse opal with uniform macroporous structure fabricated from PMMA opal is shown in Fig. 1B. The size of the macropores is ca. 410 nm, ~11% smaller than the original PMMA sphere diameter due to the volume shrinkage during the calcination process. Fig. 1C displays an SEM image of the OMCS and it retains the ordered face-centered cubic (fcc) structure in the PMMA opal after the two-step replication procedure. However, due to the shrinkage of carbon precursor during the carbonization process, the size of the carbon spheres reduced to~380 nm. Fig. 1D reveals a typical TEM image of the OMCS, and the inset of Fig. 1D shows the higher magnification image of one mesoporous carbon sphere, in which an ordered cubic mesostructure with 7-9 nm diameter mesopores can be seen. Fig. 1E shows the TEM image of high loading (60 wt%, the actual Pt contents of the catalyst is determined to be 58.4 wt% by ICP analysis) Pt nanoparticles on OMCS (Pt/OMCS). The higher magnification TEM image (inset of Fig. 1E) of the Pt/OMCS demonstrates a dense and homogeneous dispersion of the Pt nanoparticles on the surface of the mesoporous walls. From the corresponding high-resolution TEM (HR-TEM) image (Fig. 1F), the average Pt particle size is about 1.8 nm based on 50 Pt particles randomly selected.
|
Download:
|
| Figure 1. SEM images of (A) PMMA opal, (B) silica inverse opal and (C) OMCS; TEM images of (D) OMCS (inset: higher magnification image of one mesoporous carbon sphere), (E) Pt/OMCS (inset: higher magnification image); (F) HR-TEM image of Pt/OMCS. | |
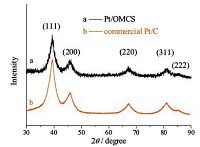
XRD (Fig. 2) was employed to further evaluate the size of the Pt particles on the OMCS. The Pt particle sizes of the Pt/OMCS and commercial 60 wt% Pt/C (HiSpec 9100, Johnson Matthey) catalysts estimated by the Scherrer equation are 2.0 and 2.6 nm, respectively. The Pt particle size of the Pt/OMCS is smaller than that of the commercial 60 wt% Pt/C and the reported high Pt loading (60 wt%) catalysts on carbon black (-3.6 nm) [25], ordered mesoporous carbon (-3.0 nm) [20], graphene aerogel (3.2 nm) [9], shell-core nanostructured carbon (3.2 nm) [26], and functionalized multiwall carbon nanotubes (-3.0 nm) [13], which is mainly attributed to the mesopore confinement effect that restricts the growth of Pt nanoparticles. Furthermore, the OMCS is more likely to adsorb precursor solution containing Pt ion than bulk mesoporous carbon powders due to its unique hierarchical nanostructure and large mesopores, which favours Pt nanoparticles evenly distributing on the mesoporous walls without large agglomerations.
|
Download:
|
| Figure 2. XRD patterns of the Pt/OMCS (a) and the commercial Pt/C (b) catalysts. | |
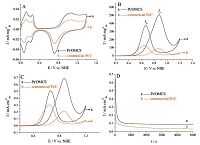
Fig. 3A shows the CV curves of Pt/OMCS and commercial Pt/C in Ar-saturated 0.5 mol L-1 H2SO4 solution at a scan rate of 50 mV s-1 at room temperature. The specific electrochemically active surface area (ECSA) of Pt was determined by the area of H desorption between 0 and 0.35 observed on CV curves, and can be calculated by the relation ECSA = QH/(m.c) [27, 28] where QH is the total charge (mC cm-2) , m is the actual Pt loading (mg cm-2) on the GC substrate, and c represents the charge required to oxidize a monolayer of hydrogen on a Pt surface (0.21 mC cm-2) . The Pt/OMCS catalyst possesses larger ECSA (73.5 m2 g-1) compared with the commercial Pt/C (43.5 m2 g-1) , which is attributed to the smaller and more densely dispersed Pt nanoparticles loaded on the OMCS. And the ECSA value of the Pt/OMCS is higher than or comparable to that of the high loading Pt (60 wt%) supported on surface-functionalized graphene nanosheets (51.0 m2 g-1) [8], graphene (42.0 m2 g-1) [29], N-doped carbon (68.0 m2 g-1) [26], ordered mesoporous carbons (66.2 m2 g-1) [30], hollow spherical carbon with mesoporous shell (62.0 m2 g-1) [18], functionalized multiwall carbon nanotubes (83.0 m2 g-1) [13], and graphene aerogel (84.1 m2 g-1) [9]. The ECSA value of the Pt/OMCS is also comparable to or larger than that of the lower loading (20 wt%) Pt nanoparticles supported on the reported mesoporous carbon materials such as the Pt/mesoporous carbon catalyst (82.5 m2 g-1) [31], the Pt/ordered mesoporous carbon sphere array catalyst (85.7 m2 g-1) [21], and the Pt/highly ordered mesoporous carbons catalyst (40.0 m2 g-1) [32].
|
Download:
|
| Figure 3. (A) CVs of Pt/OMCS (a) and commercial Pt/C (b) catalysts in 0.5 mol L-1 H2SO4; (B) CVs and (C) ECSA-normalized CVs of the catalysts in 0.5 mol L-1 H2SO4 and 1 mol L-1 CH3OH; (D) chronoamperomograms for methanol oxidation reaction catalyzed by Pt/OMCS (a) and commercial Pt/C (b) in 0.5 mol L-1 H2SO4 and 1 mol L-1 CH3OH. The scan rate of the CVs is 50 mV s-1, and the chronoamperomograms were recorded at 0.8 V. | |
CVs of the catalysts toward methanol oxidation obtained in 0.5 mol L-1 H2SO4 and 1 mol L-1 CH3OH solution at a scan rate of 50 mV s-1 at room temperature are shown in Fig. 3B and C. The peak current densities of the Pt/OMCS and commercial Pt/C catalysts during the forward scan reach 510 mA mg-1 (0.69 mA cm-2) and 92 mA mg-1 (0.22 mA cm-2) , respectively. The methanol oxidation mass current density of the Pt/OMC is 5.5-fold higher than that of the commercial Pt/C, and this change is greater than those reported for the Pt/mesoporous carbon materials with lower Pt loadings, such as the Pt/ordered mesoporous carbon spheres (671 mA mg-1) [21], the Pt/nitrogen/ sulfur dual-doped mesoporous carbon (505 mA mg-1) [33], and the Pt/boron-doped ordered mesoporous carbon (511 mA mg-1) [34] (4.6-, 1.7-, and 1.7-fold increases in current density, respectively, compared with the commercial Pt/C). In addition, the ratio of If (forward anodic current peak) to Ib (reverse anodic current peak) can be used to infer the poisoning tolerance of the catalyst. The Pt/OMCS shows a higher value of If/Ib (1.38) than the commercial Pt/C (0.72) , indicating an improved poison tolerance performance of the Pt/OMCS catalyst. The If/Ib value of the Pt/OMCS is comparable to or higher than that of the reported low metal-loading Pt/mesoporous carbon materials, such as the Pt/ordered mesoporous carbon spheres (1.56) [21], the Pt/ mesoporous carbon nanofibers (1.23) [35], the Pt/highly ordered mesoporous carbon nanofiber arrays (1.20) [36], and the Pt/ ordered mesoporous carbon FDU-15 (0.98) [37].
The chronoamperomograms (Fig. 3D) recorded at 0.8 V indicate that the current density of the Pt/OMCS is higher than that of the commercial Pt/C catalyst over the entire time range, also exhibiting the improved electrocatalytic performance of the Pt/OMCS catalyst for MOR. In addition, the long-term poisoning rate (d) is calculated by measuring the linear decay of the current for a period of more than 500 s from Fig. 3D by using the following equation: [38-40]
| $\delta {\rm{ = }}{{100} \over {{I_0}}} \times {\left( {{{{\rm{d}}I} \over {{\rm{d}}t}}} \right)_{t > 500s}}\left( {\% {s^{ - 1}}} \right)$ |
where (dI/dt)t>500s is the slope of the linear portion of current decay and I0 is the current at the start of polarization back extrapolated from the linear current decay. The poisoning rates are calculated to be 0.0019 and 0.0104% s-1 for Pt/OMCS and commercial Pt/C catalysts, respectively. The result further reveals the better poisoning tolerance of the Pt/OMCS catalyst.
Compared with the commercial Pt/C, the significantly enhanced electrocatalytic performance of the Pt/OMCS is probably related to the better dispersion of the Pt nanoparticles and the unique ordered interconnected marcopores and mesopores of the OMCS which allow the facile diffusion of reactants during the reactions. The MOR performance of the Pt/OMCS is higher than or comparable to that of the reported Pt/mesoporous carbon catalysts with low Pt loadings, suggesting that the Pt/OMCS, even with a high Pt loading, would be one of the most active MOR catalysts.
4. ConclusionIn summary, OMCS has been explored to support high loading Pt nanoparticles (60 wt%) as highly efficient electrocatalysts for methanol oxidation reaction (MOR). The prepared Pt/OMCS exhibits uniformly dispersed Pt nanoparticles with an average particle size of ~2.0 nm on the mesoporous walls of the OMCS. The Pt/OMCS catalyst shows larger ECSA and higher MOR performance in comparison to the commercial Pt/C, which can be attributed to the improved dispersion of Pt nanoparticles and the fantastic hierarchical nanostructure with interconnected ordered macropores and mesopores that ensure fast mass transport during the reactions. The high metal-loading Pt/OMCS catalyst with great MOR performance is very important for reducing the diffusion layer thickness and mass transport resistance in electrodes; therefore, this system has potential as an electrocatalyst in the fabrication of thin-layered MEAs.
AcknowledgmentThe authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 51172014) , the National 973 Program of China (No. 2009CB219903) , and the Scientific Innovation Grant for Excellent Young Scientists of Hebei University of Technology (No. 2015001) .
| [1] | X.L. Li, A. Faghri. Review and advances of direct methanol fuel cells (DMFCs) part I: design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 226 (2013) 223–240. |
| [2] | X. Zhao, M. Yin, L. Ma, et al. , Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 4 (2011) 2736–2753. |
| [3] | J.N. Tiwari, R.N. Tiwari, G. Singh, K.S. Kim. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2 (2013) 553–578. |
| [4] | H.S. Liu, C.J. Song, L. Zhang, et al. , A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 155 (2006) 95–110. |
| [5] | H.J. Huang, X. Wang. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2 (2014) 6266–6291. |
| [6] | S. Sharma, B.G. Pollet. Support materials for PEMFC and DMFC electrocatalysts-a review. J. Power Sources 208 (2012) 96–119. |
| [7] | J. Zhao, W.X. Chen, G. Han, Y.F. Zheng, Z.D. Xu. Platinum nanoparticles supported on graphite nanofibers prepared by microwave irradiation and its electrocatalytic activity. Chin. Chem. Lett. 16 (2005) 269–272. |
| [8] | S.M. Choi, M.H. Seo, H.J. Kim, W.B. Kim. Synthesis of surface-functionalized graphene nanosheets with high Pt-loadings and their applications to methanol electrooxidation. Carbon 49 (2011) 904–909. |
| [9] | Q.H. Huang, F.F. Tao, L.L. Zou, et al. , One-step synthesis of Pt nanoparticles highly loaded on graphene aerogel as durable oxygen reduction electrocatalyst. Electrochim. Acta 152 (2015) 140–145. |
| [10] | L. Kaluža, M.J. Larsen, M. Zdražil, et al. , Highly loaded carbon black supported Pt catalysts for fuel cells. Catal. Today 256 (2015) 375–383. |
| [11] | D.J. You, K. Kwon, S.H. Joo, et al. , Carbon-supported ultra-high loading Pt nanoparticle catalyst by controlled overgrowth of Pt: improvement of Pt utilization leads to enhanced direct methanol fuel cell performance. Int. J. Hydrogen Energy 37 (2012) 6880–6885. |
| [12] | G.S. Chai, B.Z. Fang, J.S. Yu, g-Ray irradiation as highly efficient approach for synthesis of supported high Pt loading cathode catalyst for application in direct methanol fuel cell, Electrochem. Commun. 10 (2008) 1801-1804. |
| [13] | B.Z. Fang, M.S. Kim, J.H. Kim, et al. , High Pt loading on functionalized multiwall carbon nanotubes as a highly efficient cathode electrocatalyst for proton exchange membrane fuel cells. J. Mater. Chem. 21 (2011) 8066–8073. |
| [14] | J.H. Kim, D.S. Kim, H.H. Chun, Y.T. Kim. Direct covalent thiolation of carbon nanotube supports to enhance the durability of highly loaded Pt electrocatalysts. Electrochem. Commun. 19 (2012) 85–89. |
| [15] | J.Y. Cao, C. Du, S.C. Wang, et al. , The production of a high loading of almost monodispersed Pt nanoparticles on single-walled carbon nanotubes for methanol oxidation. Electrochem. Commun. 9 (2007) 735–740. |
| [16] | M.S. Saha, R. Li, X.L. Sun. High loading and monodispersed Pt nanoparticles on multiwalled carbon nanotubes for high performance proton exchange membrane fuel cells. J. Power Sources 177 (2008) 314–322. |
| [17] | S.H. Joo, S.J. Choi, I. Oh, et al. , Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412 (2001) 169–172. |
| [18] | B.Z. Fang, J.H. Kim, M. Kim, M. Kim, J.S. Yu. Hierarchical nanostructured hollow spherical carbon with mesoporous shell as a unique cathode catalyst support in proton exchange membrane fuel cell. Phys. Chem. Chem. Phys. 11 (2009) 1380–1387. |
| [19] | J.H. Kim, B.Z. Fang, S.B. Yoon, J.S. Yu. Hollow core/mesoporous shell carbon capsule as an unique cathode catalyst support in direct methanol fuel cell. Appl. Catal. B: Environ. 88 (2009) 368–375. |
| [20] | S.H. Joo, K. Kwon, D.J. You, et al., Preparation of high loading Pt nanoparticles on ordered mesoporous carbon with a controlled Pt size and its effects on oxygen reduction andmethanol oxidation reactions, Electrochim.Acta 54(2009) 5746-5753. |
| [21] | C.W. Zhang, L.B. Xu, N.N. Shan, et al. , Enhanced electrocatalytic activity and durability of Pt particles supported on ordered mesoporous carbon spheres. ACS Catal. 4 (2014) 1926–1930. |
| [22] | R.C. Schroden, M. Al-Daous, C.F. Blanford, A. Stein. Optical properties of inverse opal photonic crystals. Chem. Mater. 14 (2002) 3305–3315. |
| [23] | Y. Meng, D. Gu, F.Q. Zhang, et al. , Ordered mesoporous polymers and homologous carbon frameworks: amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 44 (2005) 7053–7059. |
| [24] | H.J. Liu, W.J. Cui, L.H. Jin, C.X. Wang, Y.Y. Xia. Preparation of three-dimensional ordered mesoporous carbon sphere arrays by a two-step templating route and their application for supercapacitors. J. Mater. Chem. 19 (2009) 3661–3667. |
| [25] | S.S. Li, H.Y. Liu, Y. Wang, et al. , Controlled synthesis of high metal-loading. Pt-based electrocatalysts with enhanced activity and durability toward oxygen reduction reaction. RSC Adv. 5 (2015) 8787–8792. |
| [26] | G. Wu, D.Y. Li, C.S. Dai, D.L. Wang, N. Li. Well-dispersed high-loading Pt nanoparticles supported by shell-core nanostructured carbon for methanol electrooxidation. Langmuir 24 (2008) 3566–3575. |
| [27] | H.A. Gasteiger, S.S. Kocha, B. Sompalli, F.T. Wagner. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B: Environ. 56 (2005) 9–35. |
| [28] | T.R. Ralph, G.A. Hards, J.E. Keating, et al. , Low cost electrodes for proton exchange membrane fuel cells: performance in single cells and Ballard stacks. J. Electrochem. Soc. 144 (1997) 3845–3857. |
| [29] | M.H. Seo, S.M. Choi, H.J. Kim, W.B. Kim. The graphene-supported Pd and Pt catalysts for highly active oxygen reduction reaction in an alkaline condition. Electrochem. Commun. 13 (2011) 182–185. |
| [30] | S.H. Joo, C. Pak, D.J. You, et al. , Ordered mesoporous carbons (OMC) as supports of electrocatalysts for direct methanol fuel cells (DMFC): effect of carbon precursors of OMC on DMFC performances. Electrochim. Acta 52 (2006) 1618–1626. |
| [31] | S. Vengatesan, H.J. Kim, S.K. Kim, et al. , High dispersion platinum catalyst using mesoporous carbon support for fuel cells. Electrochim. Acta 54 (2008) 856–861. |
| [32] | S.Q. Song, K. Wang, Y.H. Liu, et al. , Highly ordered mesoporous carbons as the support for Pt catalysts towards alcohol electrooxidation: the combined effect of pore size and electrical conductivity. Int. J. Hydrogen Energy 38 (2013) 1405–1412. |
| [33] | Y.Q. Chang, F. Hong, J.X. Liu, et al. , Nitrogen/sulfur dual-doped mesoporous carbon with controllable morphology as a catalyst support for the methanol oxidation reaction. Carbon 87 (2015) 424–433. |
| [34] | A. Nsabimana, X.J. Bo, Y.F. Zhang, et al. , Electrochemical properties of borondoped ordered mesoporous carbon as electrocatalyst and Pt catalyst support. J. Colloid Interface Sci. 428 (2014) 133–140. |
| [35] | G.W. Zhao, J.P. He, C.X. Zhang, et al. , Highly dispersed Pt nanoparticles on mesoporous carbon nanofibers prepared by two templates. J. Phys. Chem. C 112 (2008) 1028–1033. |
| [36] | H.J. Liu, X.M. Wang, W.J. Cui, et al. , Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 20 (2010) 4223–4230. |
| [37] | D.H. Lin, Y.X. Jiang, S.R. Chen, S.P. Chen, S.G. Sun. Preparation of Pt nanoparticles supported on ordered mesoporous carbon FDU-15 for electrocatalytic oxidation of CO and methanol. Electrochim. Acta 67 (2012) 127–132. |
| [38] | J.H. Jiang, A. Kucernak. Electrooxidation of small organic molecules on mesoporous precious metal catalysts I: CO and methanol on platinum. J. Electroanal. Chem. 533 (2002) 153–165. |
| [39] | J.H. Jiang, A. Kucernak. Electrooxidation of small organic molecules on mesoporous precious metal catalysts: II: CO and methanol on platinum-ruthenium alloy. J. Electroanal. Chem. 543 (2003) 187–199. |
| [40] | D. Basu, S. Basu. Synthesis and characterization of Pt-Au/C catalyst for glucose electro-oxidation for the application in direct glucose fuel cell. Int. J. Hydrogen Energy 36 (2011) 14923–14929. |
 2016, Vol. 27
2016, Vol. 27 


