b College of Chemical Science and Technology, Yunnan University, Kunming 650091, China ;
c College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650500, China
Mercury is one of the most toxic heavy metals found in aquatic systems, and it has become a worldwide issue in recent years due to its severe risk to human health and to the environment. Compared with many of the current techniques for mercury detection, such as atomic absorption spectrometry, inductively coupled plasma mass spectroscopy, spectrophotometry, neutron activation analysis, anodic stripping voltammetry and X-ray fluorescence spectrometry, fluorescence sensing provide simple, safe, effective and rapid detection of Hg2+, paving the way for its applications [1]. Even a number of research groups have reported their recent achievements on important ion-targeted or aniontargeted fluorescent sensors [2], the development of organic probes for sensing of environmentally hazardous Hg2+ ions is still of great importance due to their implications in broad areas.
Herein, we have designed and synthesized a simple and easy to prepare fluorescent chemosensor (1) for the Hg2+ ion sensing. In molecule 1, rhodamine group acts as a fluorophore group, with a hydroxyquinoline group as a recognition group, allowing the coordination capacity required to chelate mercury ions. Sensor 1 showed colorimetric and fluorescent selectivity for Hg2+ in DMSO- HEPES buffer (0.02 mol/L, pH 7.4; v/v = 6:4) solution over other common physiologically important metal ions. It showed that sensor 1 was a low toxic compound, and was successfully applied in the in vivo imaging of Hg2+ in Spill 2 cells and C. elegans.
2. Experimental 2.1. Reagents and chemicalsAll reagents were purchased from commercial sources and were used without further purification. Flash chromatography was carried out on silica gel (230-400 mesh). 1H NMR spectra (DMSOd6) were recorded using Ascend TM 400 spectrometer; 13C NMR spectra (DMSO-d6) were recorded using Avance Ⅲ 400 spectrometer; mass spectrometry was recorded with Xevo TQ-S mass spectrometer. The UV-vis spectrum was obtained using UV- 240IPC spectrophotometer. The fluorescence spectra were obtained with F-4500 FL spectrometer with a 1 cm standard quartz cell. The cells were imaged using an Olympus IX71 inverted fluorescence microscopy. The mounted nematodes were imaged using an Olympus BX51 inverted fluorescence microscopyll reagents were of analytical grade or the best grade commercially available, and were put into use without further purification. Deionized water was used throughout. HEPES buffer solutions (0.02 mol/L, pH 7.4) were prepared in deionized water. Analyte solutions of the perchlorate of Al3+, Na+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Cd2+, Ag+, Fe3+, Hg2+ and Cr3+ were prepared by dissolving the salts in distilled water to final concentrations of 0.1 mol/L.
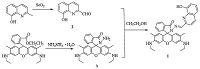
2.2. Synthesis of probe 1Compound 2 was synthesized from 2-methyloxine by the procedure published in literature [3]. Compound 3, as a known rhodamine 6G derivative, was synthesized as described previously [4]. Compounds 2 (210 mg, 1.2 mmol) and 3 (0.307 mg, 0.8 mmol) were mixed in boiling ethanol with three drops of acetic acid (Scheme 1). After 8 h of stirring, the pink precipitate formed was removed by filtration, washed with ethanol/diethyl ether (1:1), and purified by silica gel column chromatography to afford orange-yellow solid product 1 (230 mg, =%). 1 H NMR (400 MHz, DMSO-d6): 9.87 (s, 1H), 8.69 (s, 1H), 8.24-8.22 (d, 1H, J = 8.8 Hz), 7.98-7.96 (d, 1H, J = 7.2 Hz), 7.87-7.85 (d, 1H, J = 8.8 Hz), 7.62-7.57 (m, 2H), 7.40-7.38 (d, 2H, J = 7.6 Hz), 7.33-7.31 (d, 1H, J = 8.0 Hz), 7.09-7.0-5 (t, 2H, J = 7.2 Hz), 6.40 (s, 2H), 6.27 (s, 2H), 5.10 (s, 2H), 3.17-3.12 (m, 4H), 1.85 (s, 6H), 1.23-1.19 (t, 6H, J = 7.0 Hz); 13C NMR (100 MHz, DMSO-d6): 168.87, 153.90, 152.51, 152.39, 151.26, 148.35, 145.98, 138.54, 137.00, 134.80, 129.29, 129.24, 128.71, 127.96, 126.93, 124.16, 123.82, 118.86, 118.26, 117.46, 112.79, 10-5.10, 96.58, 66.02, 32.00, 17.44, 14.64. HRMS (ESI, m/z): calcd. for C36H33N5O3 584.2583 [M+H]+, 606.2476 [M+Na]+, found 584.2650 [M+H]+; 606.2477 [M+Na]+.
|
Download:
|
| Scheme. 1. Synthetic route of probe 1. | |
3. Results and discussion
In order to clarify the interaction of 1 with metal ions, the UV- vis absorption spectra of 1 were first studied in DMSO-HEPES buffer (0.02 mol/L, pH 7.4, v/v = 6:4) solutions. Cd2+, Ni2+, Zn2+, Fe3+, Cr3+, Al3+, Ag+, Co2+, Cu2+, and Hg2+ were used to measure the selectivity of probe 1. All spectra were recorded after three minutes upon addition of 25 equiv. of each of these ions. As shown in Fig. 1, compound 1 exhibits no major absorption band. Upon addition of different metal ions, only the presence of Hg2+ could lead to an obvious absorption increase at 533 and 662 nm (Fig. 1). It revealed that 1 had a good selectivity toward Hg2+ in the absorption among the tested ions. In the titration tests, with the addition of Hg2+, the absorbance at 533 and 662 nm increased sharply, which induced a color change from colorless to pink (Fig. 2c).

|
Download:
|
| Figure 1. (a) Absorption spectra of 1 (2.0 × 10-5 mol/L) in DMSO–HEPES buffer (0.02 mol/L, pH 7.4, v/v = 6:4) with 25 equiv. of Fe3+, Cr3+, Co2+, Ni2+, Na+, Al3+, Cd2+, Zn2+, Hg2+; (b) Absorbances of 1 (2.0 × 10-5 mol/L) at 533 nm after addition of 25 equiv. selected ions (1: blank, a: Zn2+, b: Ni2+, c: Cd2+, d: Fe3+, e: Al3+, f: Na+, g: Hg2+, h: Cr3+, i: Co2+). | |

|
Download:
|
| Figure 2. (a) Absorption titration spectra of 1 (2.0 × 10-5 mol/L) in the presence of varying concentrations of Hg2+ in DMSO–HEPES buffer (0.02 mol/L, pH 7.4; v/v = 6:4). (b) Absorbance of 1 (2.0 × 10-5 mol/L) at 533 nm as a function of varying concentrations of Hg2+. (c) Picture of compound 1 (left) and compound 1 upon the addition of Hg2+ (50 × 10-5 mol/L) (right). | |
Then, Cd2+, Ni2+, Zn2+, Na+, Fe3+, Cr3+, Al3+, Pb2+, Ag+, Co2+, Cu2+ and Hg2+ were used to evaluate fluorescent selectivity of compound 1 in DMSO-HEPES buffer (0.02 mol/L, pH 7.4, v/v = 6:4) solutions. From the fluorescence experiments (Fig. 3a), clear ‘‘off-on’’ fluorescence changes of 1 to Hg2+ were observed. Among the tested metal ions (30 equiv.), 1 showed a selective fluorescence enhancement only with Hg2+, indicating that 1 displayed a high Hg2+ selectivity (Fig. 3b).

|
Download:
|
| Figure 3. (a) Fluorescence emission spectra of 1 (2.0 × 10-5 mol/L) in DMSO–HEPES buffer (0.02 mol/L, pH 7.4, v/v = 6:4) with 30 equiv. of Hg2+; (b) fluorescence intensity of 1 (2.0 × 10-5 mol/L) at 556 nm after addition of 30 equiv. selected ions. (1: blank, a: Cd2+, b: Ni2+, c: Zn2+, d: Na+, e: Fe3+, f: Cr3+, g: Al3+, h: Pb2+, i: Ag+, j: Co2+, k: Cu2+, l: Hg2+). | |
Compound 1 displays almost no fluorescence. When Hg2+ was added to the solution, a significant increase of the fluorescence intensity of =6 nm, which was attributed to the Hg2+-induced ring opening of the spirolactam moiety, was observed. Hg2+ generated a significant fluorescence enhancement of up to 300-fold, with a bright yellow-green emission (Fig. 4). These results suggested that 1 has high fluorescence selectivity for Hg2+ compared to the other ions.

|
Download:
|
| Figure 4. (a) Fluorescence titration spectra of 1 (2.0 × 0-5 mol/L) in the presence of varying concentrations of Hg2+ in DMSO–HEPES buffer (0.02 mol/L, pH 7.4; v/v = 6:4). Excitation wavelength was 528 nm. (b) Fluorescence intensity of 1 (2.0 × 0-5 mol/L) at 556 nm as a function of varying concentrations of Hg2+. (c) Fluorescence picture of compound 1 (left) and compound 1 upon the addition of Hg2+ (50 × 10-5 mol/L) (right). | |
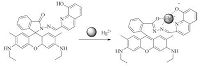
The proposed binding mechanism of compound 1 with Hg2+ is shown in the Scheme 2. The spirolactam moiety of the rhodamine group acts as a signal switcher, when 1 binds Hg2+, the fluorescence-off state of 1 converts to the Hg2+-promoted ringopened amide form with a fluorescence-on state. Moreover, 1 is most likely to bind Hg2+ via the imide N and quinoline O atoms like other reported researches [5]. The detection limit was calculated based on the method reported in the previous literature [6]. The fluorescence intensity at =6 nm was plotted as a concentration of Hg2+, and detection limit was calculated to be 6.79 × 10-6 L/mol by using detection limit 3σ/k: Where σ is the standard deviation of blank measurement, k is the slope between the fluorescence intensity versus Hg2+ concentration.
|
Download:
|
| Scheme. 2. The proposed binding mechanism of compound 1 with Hg2+. | |
|
Download:
|
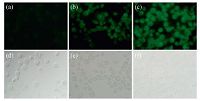
| Figure 5. Fluorescent imaging (top) and phase contrast (bottom) for Hg2+ in Spill 2 cells. (a) Probe 1 (2.0 × 10-5 mol/L) only. (b) Probe 1 (2.0 × 10-5 mol/L) and Hg2+ (30 × 10-5 mol/L). (c) Probe 1 (2.0 × 10-5 mol/L) and Hg2+ (60 × 10-5 mol/L). | |
To investigate the cytotoxicity of compound 1, CCK8 tests were performed in HaCaT cells, L6 cells, MA104 cells, RAEC cells, Ins- 1cells, HepG2 cells, A549 cells and SKOV cells. When 50% of same type experimental cells produce a particular response in specific concentration of 1, the corresponding concentration is EC50 (effective concentration). In this experiment, EC50 is used to represent the cytotoxicity of compound 1 toward cells. As shown in Table S1 in Supporting information, it proved that 1 is low toxic and safe for the in vivo tests.
To demonstrate the feasibility of 1 for its application in in vivo imaging, fluorescence imaging tests were performed in Spill 2 cells and C. elegans. Previously, the author successfully designed a pyrene-bearing probe to test and evaluate the toxicity levels of heavy metal mercury in BEAS-2B cells, CHO cells, C. elegans, and zebrafish [7]. In this study, the application of 1 in in vivo imaging was first evaluated by visualizing the distribution of Hg2+ in cells incubated with different amount of Hg2+ for 2 h. As shown in Fig. 5, with the dose of Hg2+ was increased, fluorescence emission became brighter and brighter. Green fluorescence emission colors were mainly observed in the cell cytoplasm, whereas the cell nucleus showed weak fluorescence. This suggests that the 1-Hg2+ agent showed a good bonding capacity for the cytoplasm and a weak binding capacity for nucleic acids in the tested cells [8].
The application of 1 in in vivo imaging was evaluated by visualizing the distribution of Hg2+ in nematodes previously incubated with various concentrations of Hg2+ for 5 h. C. elegans larvae at developmental stage 4 (L4) were then incubated in Petri dishes filled with M9 buffer, which contained 1 (20 mmol/L) at 20 oC for 1 h. Almost no fluorescence could be observed, when the concentration of Hg2+ is around 300 mmol/L (Fig. 6b). Very weak fluorescence was observed in the pretreated nematodes until the concentration of Hg2+ reached 600 mmol/L (Fig. 6c). The abovementioned investigations confirmed that 1 could stain and sense Hg2+ in C. elegans.

|
Download:
|
| Figure 6. Fluorescent imaging (top) and phase contrast (bottom) for Hg2+ in C. elegans. (a) with 2.0 × 10-5 mol/L of 1 only; (b) with 30 × 10-5 mol/L Hg2+ for 5 h and 2.0 × 10-5 mol/L of 1 for 1 h; (c) with 60 × 10-5 mol/L Hg2+ for 5 h and 2.0 × 10-5 mol/L of 1 for 1 h. | |
4. Conclusion
In conclusion, a rhodamine derivative, 1, was synthesized as a fluorescent sensor for Hg2+, with a binding process marked by a distinct ‘‘off/on’’ fluorescent change. Hg2+- induced ring opening of the spirolactam moiety and generated a significant fluorescence enhancement of up to 300-fold, with a bright yellow-green emission. Compound 1 was a low toxic compound, and was successfully applied in the in vivo imaging of Hg2+ in Spill 2 cells and C. elegans, which supported its further utility in environmental and tobacco samples.
AcknowledgmentsThis work was supported by the fund of China Tobacco Yunnan Industrial Co. (No. 2015JC0-5), the Foundation of the Department of Science and Technology of Yunnan Province of China (Nos. 2013HB062, 2014HB008), and Training Project (No. XT412003) of Yunnan University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.04.001.
| [1] |
(a) K. Bera, A.K. Das, M. Nag, S. Basak, Development of a rhodamine-rhodaninebased fluorescent mercury sensor and its use to monitor real-time uptake and distribution of inorganic mercury in live zebrafish larvae, Anal. Chem. 86 (2014) 2740-2746; (b) Y. Zhu, H. Li, B. Shi, et al., A reversible fluorescent chemosensor for the rapid detection of mercury ions (Ⅱ) in water with high sensitivity and selectivity, RSC Adv. 4 (2014) 61320-61323; (c) Y. Liu, E.B. Yang, R. Han, et al., A new rhodamine-based fluorescent chemosensor for mercury in aqueous media, Chin. Chem. Lett. 25 (2014) 1065-1068; (d) Z. Wang, Z. Yang, T. Gao, et al., An Hg2+-selective chemosensor based on the self-assembly of a novel amphiphilic block copolymer bearing rhodamine 6G derivative moieties in purely aqueous media, Anal. Methods 7 (2015) 2738-2746. |
| [2] |
(a) J.F. Zhang, Y. Zhou, J. Yoon, J.S. Kim, Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions), Chem. Soc. Rev. 40 (2011) 3416-3429; (b) Y. Zhou, J.F. Zhang, J. Yoon, Fluorescence and colorimetric chemosensors for fluoride-ion detection, Chem. Rev. 114 (2014) 5511-5571; (c) N. Kumar, V. Bhalla, M. Kumar, Resonance energy transfer-based fluorescent probes for Hg2+, Cu2+ and Fe2+/Fe3+ ions, Analyst 139 (2014) 543-558. |
| [3] |
(a) L. Huang, F. Hou, J. Cheng, et al., Selective off-on fluorescent chemosensor for detection of Fe3+ ions in aqueous media, Org. Biomol. Chem. 10 (2012) 9634-9638; (b) N.R. Chereddy, K. Saranraj, A.K. Barui, et al., Donor atom selective coordination of Fe3+ and Cr3+ trigger fluorophore specific emission in a rhodamine-naphthalimide dyad, RSC Adv. 4 (2014) 24324-24327. |
| [4] | (a) L.E. Guo, X.Y. Liu, H. Wang, et al., Iron(Ⅲ)-selective chelation-enhanced fluorescence sensing for in vivo imaging applications, Chem. Asian J. 10 (2015) 1898-1902. |
| [5] | Z. Dong, X. Tian, Y. Chen, et al. , A highly selective fluorescent chemosensor for Hg2+ based on rhodamine B and its application as a molecular logic gate. Dyes Pigm. 97 (2013) 324–329. |
| [6] | B. Zhu, C. Gao, Y. Zhao, et al. A 4-hydroxynaphthalimide-derived ratiometric fluorescent chemodosimeter for imaging palladium in living cells. Chem. Commun. 47 (2011) 8656–8658. |
| [7] | G.K. Wang, Q.L. Mi, L.Y. Zhao, et al. , A pyrene derivative for Hg2+-selective fluorescent sensing and its application in in vivo imaging. Chem. Asian J. 9 (2014) 744–748. |
| [8] | L.Y. Zhao, Q.L. Mi, G.K. Wang, et al. 1, 8-Naphthalimide-based ‘turn-on' fluorescent sensor for the detection of zinc ion in aqueous media and its applications for bioimaging. Tetrahedron Lett. 54 (2013) 3353–3358. |
 2016, Vol. 27
2016, Vol. 27 


