b. School of Chemistry and Environmental Engineering, Wuhan Institute of Technology, Wuhan 430073, China
Developing green, efficient and advanced energy storage and conversion systems, such as supercapacitors and lithium-ion batteries, have attracted much attention during the past several years [1]. Supercapacitors show long cycle life, high specific power and the capability to bridge the power/energy gap between conventional electrostatic capacitors and batteries/fuel cells [2]. Consequently, supercapacitors are currently considered as one of the most promising energy-storage and power-supply devices for portable electronics, backup or auxiliary power sources in pure and hybrid electric vehicles, large industrial equipments, as well as other renewable energy storage systems [3]. Based on the energy storage mechanism, supercapacitors can be classified into two categories: (1) Electric double-layer capacitors (EDLCs) which store energy at the electrode/electrolyte interface by charging the "double-layer capacitance" through reversible ion adsorption on the electrode surface [4], and (2) Pseudocapacitors where the capacitance arises from a fast and reversible faradaic redox reaction at electro-active materials, such as transition metal oxides and conducting polymers [5].
Using carbon materials as the electrode active materials, EDLCs represent today more than 80% of the commercially manufactured supercapacitors [6]. According to the energy storage principle, high specific surface area of electro-active materials is a basic requirement for EDLCs to achieve large electrochemical capacitance. Microporous carbons, especially activated carbons, show high capability for charge accumulation at the electrolyte/carbon interface due to their large surface area, good electronic conductivity and superior chemical stability, and thus are considered as preferred electrode materials for supercapacitors [7]. Unfortunately, most activated carbons suffer from the disadvantage of very small and island-like micropores which do not allow the fast transfer of electrolyte ions into the carbon surface, and thus cause much difficulty to achieve rapid formation of a double-layer, especially under a large current density charge/ discharge process [8]. On the contrary, mesoporous carbon materials with well structured and large mesopores are favorable for high transportation speed of electrolyte ions, and thus exhibit much higher rate performance [9]. However, large mesopore is generally in contradiction with high surface area, and consequently leads to insufficient specific capacitance of the final EDLC devices.
Microporous carbons can be used in a better way in supercapacitor electrodes if their extremely small and island-like micropores are optimized properly, aiming at reaching the target where fast and efficient ion diffusion pathway become a reality and high surface area is maintained [3a,8,10]. For example, Xu group employed metal-organic framework with three-dimensional intersecting channel as a template and furfuryl alcohol as carbon source to fabricate nanoporous carbons which show good electrochemical performance as an EDLC electrode (258 F/g at 250 mA/g) [10a]. Itoi et al. demonstrated the synthesis of zeolitetemplated ordered microporous carbons with regularly arrayed micropores (1.2 nm) which exhibits a gravimetric capacitance (140-190 F/g) in an organic electrolyte solution at a high current density of 20 A/g [10c]. It is also reported by Xu et al. and our group that ultramicroporous carbons with a regular pore size of ~0.6 nm exhibit excellent electrochemical performance for supercapacitor applications [3a,8,10d,e].
Zinc ions can form a complex with oxygen-containing organic ligands, in particular with tartaric acid [10f]. Considering this chelating property, herein, we report a novel hydrothermal synthesis of microporous carbons toward electrochemical energy storage using zinc-organic complex as a template and resorcinol/ formaldehyde (R/F) resol as a carbon source. Our strategy is to make homogeneous dispersity of zinc tartrate into the networks of R/F resol. The zinc-containing R/F resol was hydrothermal polymerized, followed by carbonization to decompose the polymer and evaporate zinc template. This method achieves large mean pore size (1.99 nm) without compromising the high specific surface area (up to 1255 m2/g) in the resultant carbons, which is beneficial for high specific capacitance (225 F/g at 1.0 A/g), high rate capability (fast charge/discharge under a high current density of 20 A/g) as well as good cycle stability (93% capacitance retention at 1.0 A/g after 1000 cycles) of the carbon electrode in 6 mol/L KOH electrolyte. The result presented in this work provides new opportunities to fabricate well-designed microporous carbons for high-performance supercapacitor applications.
2. ExperimentalResorcinol, formaldehyde solution (37-40 wt%), HCl (37 wt%), tartaric acid, ZnCl2, Na2CO3, and KOH were analytical reagents purchased from Sinopharm Chemical Reagent Co., Ltd. Polytetrafluoroethylene (PTFE, catalog No. FR301B) was supplied by Shanghai 3F New Materials Co., Ltd. Nickel foams were purchased from Shanghai Hongxiang Screen Factory. Pure nitrogen was purchased from Shanghai BOC Special Gases Sales Service Co., Ltd. All chemicals were used as received without any further purification.
1.1 g of resorcinol (10mmol), 1.5 mL of formaldehyde (20mmol), 13.9mL of sodium carbonate solution (1.0 wt%) were mixed with 87 mL of distilled water. The mixed solutionwas heated to 85 ℃ for 180 min to obtain R/F resol. 2.25 g of DL-tartaric acid (15mmol), 2.0 g of zinc chloride (15mmol) and 8.3mL of hydrochloric acid (37 wt%) were mixed with 27.8 mL of H2O to obtain zinc tartrate complex, as shown in Fig. 1. This zinc-organic complex solutionwas added into R/F resol and then the mixture was transferred into a Teflon autoclave at 100-130 ℃ for 24 h. Then the autoclave was cooled naturally to roomtemperature, the resultant zinc-containing R/F polymer was dried, followed by carbonization at =0 ℃ for 30 min and then 950 ℃ (5 ℃/min) for 8 h under N2 flow to decompose the polymer and evaporate zinc template, and to fabricate microporous carbons (denoted as MC-x where x represents the hydrothermal temperature).

|
Download:
|
| Fig. 1.Formation of zinc tartrate complex. | |
Powder X-ray diffraction (XRD) was conducted on an X-ray diffractometer (Bruker AXS, D8 Advance) with Cu Kα radiation (λ = 0.15418 nm). N2 adsorption and desorption analysis was measured at -196 ℃ on a MircomeriticsTristar 3000 porosimeter. Specific surface area was calculated by Brunauer-Emmett-Teller (BET) method using adsorption data, and pore volume and pore size were derived from the adsorption branches of isotherms using the Barrett-Joyner-Halenda (BJH) model. The morphology was characterized by S-4800 scanning electron microscopy (SEM, Hitachi, Japan). Electrochemical measurements were taken on a CHI 660D electrochemical workstation using a three-electrode system. Hg/HgO electrode was used as a reference electrode, and nickel foil as a counter electrode. The working electrode was prepared by pressing the mixture of active materials (microporous carbon, 90 wt%) and PTFE (10 wt%) binder between two pieces of nickel foam under 20 MPa. Then a ~1.0 mm thick circle electrode (with a diameter of ~1.2 cm) was dried overnight at 100 ℃ to obtain the working electrode. The electrochemical performance was measured by cyclic voltammetry (CV) and galvanostatic charge-discharge (DCD) with a potential window of -1.0 V to 0 V and electrochemical impedance spectroscopy (frequency range between 1 mHz and 103 kHz) in 6 mol/L KOH solution. The specific capacitances (C, F/g) of the electrodes were calculated from GCD curves by the equation of C = IΔt/(ΔVm), where I is the discharge current, Δt is the discharge time, ΔV is the voltage difference within the discharge time Δt, and m is the mass of active materials on the working electrode.
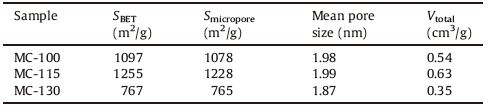
3. Results and discussionFig. 2 shows XRD patterns of MC-115 sample carbonized at different temperature. The strong and well-defined diffraction peaks of MC-115carbonized at 530 ℃are assigned to the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), and (2 0 1) lattice planes of hexagonal-phase ZnO (wurtzite structure, space group P63mc) as reported in JCPDS card (No. 36-1451) [10g]. This suggests that Zn-tartaric acid complex inside the framework of R/F polymer was decomposed to ZnO. The broad diffraction peaks at ~23° and a weak peak at ~448 correspond to the (0 0 2) and (1 0 0) lattice planes of amorphous carbon. The diffraction peaks of ZnO disappear and those of amorphous carbon remain when carbonized at 950 ℃, indicating the complete removal of Zn species and pure carbon materials were obtained. ZnO could be reduced to gaseous metal Zn by carbon via ZnO + C→Zn (g) + CO at -900 ℃ [10h].
|
Download:
|
| Fig. 2.XRD patterns of MC-115 carbonized at different temperature. | |
Fig. 3 shows N2 adsorption and desorption isotherms and pore size distribution curves of as-prepared microporous carbons. These isotherms are typical type I curve (Fig. 3a) according to the IUPAC classification, which is given by microporous solids [11]. The carbons comprise mainly micropores, as shown in Fig. 3b. Table 1 gives the pore structure parameters of the microporous carbons. The microporous carbons show BET surface areas of 767-12=m2/g and total pore volumes of 0.35-0.63 cm3/g which are mainly contributed by micropores. Zinc tartrate can be obtained through the coordination bonds between the hydroxyl groups and zinc ions. It can formhydrogen bonds with R/F resol, which is beneficial to the effective introduction and homogeneous dispersity of zinc tartrate into the framework of carbon precursor. Besides, there is also H-bonding interaction among zinc tartrate molecules, which benefits the assembly of the complex and consequent large pore size of the carbons after template removal.Hydrothermal polymerization leads to the formation of R/F polymer encapsulated zinc tartrate. During carbonization process, the decomposition of R/F polymer and the release of small molecules (mostly CO2, CO, C6H6) generate micropores [10h]. Zinc tartrate encapsulated within the carbon matrix also decomposed (to ZnO), which enlarges the micropores. Besides, the carbothermal reduction of ZnO to gaseous Zn and the associated evaporation of zinc also contribute to abundant and large micropores for the carbons. As a result, the decomposition-reduction-evaporation process endows the resultantmicroporous carbons a high surface areas and a relatively large micropores. A typical SEM image of MC-115 was shown in Fig. 4. When changing the hydrothermal condition, there are no obviously macroscopic morphological changes among MC-100, MC-115 and MC-130 samples. However, increasing the hydrothermal temperature from 100 to 115 ℃ facilitated the better dispersity of Zn-containing complex into the framework of R/F polymer, which contributes larger surface area and pore volume. Higher temperature (130 ℃) will give rise to faster polymerization of R/F which prevents zinc tartaric from well introducing into the polymer scaffolds, leading to the unsatisfied porous structure.
|
|
Table 1 Pore structure parameters of microporous carbons. |

|
Download:
|
| Fig. 3.Nitrogen adsorption-desorption isotherms and pore size distribution curves of microporous carbons. | |

|
Download:
|
| Fig. 4.A typical SEM image of MC-115 (the scale bar is 2 μm). | |
Fig. 5a shows CV curves of microporous carbon electrodes at a scan rate of 50 mV/s in 6 mol/L KOH aqueous electrolyte solution. These electrodes show quasi-rectangular shapes without any redox peaks in the whole potential window between 0 and -1 V, which indicates an ideal capacitive behavior associated with electrical double layer formation (reversible adsorption and desorption of the ions) across the surface of the carbon electrodes, and also efficient charge storage with fast ion transport in aqueous electrolyte [12]. Besides, MC-115 electrode shows largest inner integrated areas compared with MC-100 and MC-130 electrodes under the same scan rate. The specific capacitance of an electrode is in proportion to the integrated area of its CV curve [5c], and thus the electrochemical capacitance of MC-115 electrode is much higher than those of MC-100 and MC-130 electrodes due to its larger surface area. Fig. 5b exhibits CV curves of MC-115 electrode at scan rates between 100 and 500 mV/s. These curves are distorted with increasing scan rate, but still retain a rectangularlike shape even at 500 mV/s, which suggest good accessibility of electrolyte ions into the electrochemically active surfaces in quick charge/discharge operations [12a].

|
Download:
|
| Fig. 5.CV curves of microporous carbon electrodes at 50 mV/s (a) and CV curves of MC-115 electrode at various scan rates (b) in 6 mol/L KOH electrolyte solution. | |
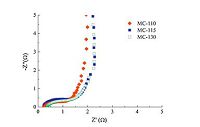
The impedance spectra analysis was conducted to understand the conductive and diffusive behavior of microporous carbon electrodes. Fig. 6 shows Nyquist plots of microporous carbon electrodes in 6 mol/L KOH electrolyte solution measured in the range of 1 mHz to 103 kHz. At low frequency, the Nyquist plots exhibit vertical lines, associated with good electrochemical capacitive properties of the electrodes [13]. Almost 45° straight lines at the intermediate frequency correspond to the characteristic of ion diffusion or transportation resistance of electrolyte into the electrode surface [14]. In the range of high frequency, the presence of the semicircles denotes polarization resistance or charge transfer resistance of the electrodes. These semicircles are not intact, indicating good charge transfer of the working electrodes [10e]. The intercept of the quasi-semicircle with the real axis reveals the equivalent series resistance (ESR), consisting of the resistance of the electrolyte solution, the intrinsic resistance of activation material, and the interfacial contact resistance of the interface active material/current collector [15]. The ESR values for MC-100, MC-115 and MC-130 electrodes are 0.30, 0.27 and 0.38 Ω, respectively.
|
Download:
|
| Fig. 6.Nyquist plots of microporous carbon electrodes in 6 mol/L KOH electrolyte solution measured in the range of 1 mHz to 103 kHz. | |
Fig. 7a gives GCD curves of microporous carbon electrodes at a loading current density of 2.0 A/g in 6 mol/L KOH electrolyte solution. The charge/discharge behavior of these carbon electrodes exhibit regularly triangular shapes with good symmetry at the whole potential window, indicating high coulombic efficiency and good reversibility. There are not obvious IR drops during the discharge process in the GCD curves, suggesting a small Ohmic resistance and good capacitive performance of the electrodes [16]. MC-115 electrode has an electrochemical capacitance of 211 F/g at a current density of 2.0 A/g, much higher than those of MC-100 (134 F/g) and MC-130 (126 F/g) electrodes under the same current density. Besides, the potential-time profile of MC-115 electrode still maintains a triangular shape even at a high current density of 20 A/g with a calculated specific capacitance of 173 F/g, as shown in Fig. 7b and c, which indicates that the carbon electrode is suitable for application in advanced supercapacitors where highrate charge/discharge process is required. Microporous carbons obtained in this work achieve high surface area to ensure high electrochemical capacitance and large pore size for rapid ion transportation to guarantee fast charge/discharge operation under large current density [17]. As a result, the MC-115 electrode takes advantage over many other carbons such as ordered microporous carbons (188 F/g at 0.5 A/g) [10a], ultramicroporous carbons (206 F/g at 1.0 A/g) [8], MOF-derived nanoporous carbons (~200 F/g at 0.5 A/g) [10e], and mesoporous carbons like KOHactivated ordered mesoporous carbons (100 F/g at 10 A/g) [18], mesoporous carbon thin film (85 F/g at 5.0 A/g) [19] and mesoporous carbon spheres (163 F/g at 20 A/g) [3c], as well as hierarchical porous carbons (~170 and 182 F/g at 10 A/g) [12c, 20]. Fig. 7d presents the cycle stability of MC-115 electrode at a loading current density of 1.0 A/g. MC-115 electrode retains 210 F/g after 1000 cycles, -93% retention of the original capacity (225 F/g), indicating good electrochemical cycling stability.

|
Download:
|
| Fig. 7.GCD curves of microporous carbon electrodes at a current density of 2.0 A/g (a), GCD curves of MC-115 electrode at different current densities (b) in 6 mol/L KOH solution, the specific capacitances of MC-115 electrode under different current densities (c), and cycle stability of MC-115 electrode at 1.0 A/g (d). | |
In conclusion, we demonstrate a novel hydrothermal synthesis of microporous carbons using zinc tartrate complex as the template and R/F resol as a carbon precursor. The resultant microporous carbons show a high specific surface area up to 1255 m2/g and a large mean pore size of 1.99 nm, which ensure high electrochemical capacitance and guarantee rapid ion transportation and fast charge/discharge operation under large current density. As a result, the microporous carbons used as supercapacitor electrodes exhibit good electrochemical performance such as high specific capacitance (225 F/g at 1.0 A/g), high rate capability (charge/discharge operation under a high current density of 20 A/g), good long-term cycle stability (~93% capacitance retention at 1.0 A/g after 1000 cycles) in 6 mol/L KOH aqueous electrolyte solution. Our methodology achieves large mean pore size without compromising the high specific surface area in microporous carbon synthesis, and thus we believe that the well-developed and high-performance microporous carbons provide new opportunities to achieve advanced supercapacitors toward high-rate electrochemical energy storage applications.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21207099, 21273162, 21473122), the Science and Technology Commission of Shanghai Municipality, China (No. 14DZ2261100), the Fundamental Research Funds for the Central Universities, and the Large Equipment Test Foundation of Tongji University.
| [1] | (a) H.W. Zhang, L. Zhou, O. Noonan, et al., Tailoring the void size of iron oxide@carbon yolk-shell structure for optimized lithium storage, Adv. Funct. Mater. 24(2014) 4337-4342;(b) G.Q. Zhang, B.Y. Xia, C. Xiao, et al., General formation of complex tubular nanostructures of metal oxides for the oxygen reduction reaction and lithium-ion batteries, Angew. Chem. (Ⅰ)nt. Ed. 52(2013) 8643-8647;(c) J.F. Ye, W. Liu, J.G. Cai, et al., Nanoporous anatase TiO2 mesocrystals:additivefree synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior, J. Am. Chem. Soc. 133(2011) 933-940;(d) H. Jiang, P.S. Lee, C.Z. Li, 3D carbon based nanostructures for advanced supercapacitors, Energy Environ. Sci. 6(2013) 41-53;(e) M.X. Liu, X.M. Ma, L.H. Gan, et al., A facile synthesis of novel mesoporous Ge@C sphere anode with stable and high capacity for lithium ion batteries, J. Mater. Chem. A 2(2014) 17107-17114;(f) J.H. Zhu, M.J. Chen, N. Yerra, et al., Microwave synthesized magnetic tubular carbon nanocomposite fabrics toward electrochemical energy storage, Nanoscale 5(2013) 1825-1830;(g) M.X. Liu, L.H. Gan, Y. Li, et al., Synthesis and electrochemical performance of hierarchical porous carbons with 3D open-cell structure based on nanosilica-embedded emulsion-templated polymerization, Chin. Chem. Lett. 25(2014) 897-901. |
| [2] | (a) Z.N. Yu, L. Tetard, L. Zhai, J. Thomas, Supercapacitor electrode materials:nanostructures from 0 to 3 dimensions, Energy Environ. Sci. 8(2015) 702-730;(b) Z.J. Fan, J. Yan, T. Wei, et al., Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density, Adv. Funct. Mater. 21(2011) 2366-2375;(c) D.Z. Zhu, Y.W. Wang, L.H. Gan, et al., Nitrogen-containing carbon microspheres for supercapacitor electrodes, Electrochim. Acta 158(2015) 166-174;(d) M.X. Liu, L.H. Gan, W. Xiong, et al., Partially graphitic micro-and mesoporous carbon microspheres for supercapacitors, Chin. Chem. Lett. 24(2013) 1037-1040. |
| [3] | (a) Y.H. Zhao, M.X. Liu, X.X. Deng, et al., Nitrogen-functionalized microporous carbon nanoparticles for high performance supercapacitor electrode, Electrochim. Acta 153(2015) 448-455;(b) J.K. Chang, M.T. Lee, W.T. Tsai, et al., Pseudocapacitive mechanism of manganese oxide in 1-ethyl-3-methylimidazolium thiocyanate ionic liquid electrolyte studied using X-ray photoelectron spectroscopy, Langmuir 25(2009) 11955-11960;(c) X.M. Ma, L.H. Gan, M.X. Liu, et al., Mesoporous size controllable carbon microspheres and their electrochemical performances for supercapacitor electrodes, J. Mater. Chem. A 2(2014) 8407-8415. |
| [4] | (a) Z.S. Wu, D.W. Wang, W.C. Ren, et al., Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors, Adv. Funct. Mater. 20(2010) 3595-3602;(b) M.X. Liu, L.H. Gan, W. Xiong, et al., Nickel-doped activated mesoporous carbon microspheres with partially graphitic structure for supercapacitors, Energy Fuels 27(2013) 1168-1173. |
| [5] | (a) Y.F. Yan, Q.L. Cheng, Z.J. Zhu, et al., Controlled synthesis of hierarchical polyaniline nanowires/ordered bimodal mesoporous carbon nanocomposites with high surface area for supercapacitor electrodes, J. Power Sources 240(2013) 544-550;(b) G. Otrokhov, D. Pankratov, G. Shumakovich, et al., Enzymatic synthesis of polyaniline/multi-walled carbon nanotube composite with core shell structure and its electrochemical characterization for supercapacitor application, Electrochim. Acta 123(2014) 151-157;(c) M.X. Liu, L.H. Gan, W. Xiong, et al., Development of MnO2/porous carbon microspheres with apartially graphitic structure for high performance supercapacitor electrodes, J. Mater. Chem. A 2(2014) 2555-2562. |
| [6] | P. Simon, Y. Gogotsi, Capacitive energy storage in nanostructured carbon electrolyte systems, Acc. Chem. Res. 46(2013) 1094-1103. |
| [7] | (a) J.S. Qian, M.X. Liu, L.H. Gan, et al., A seeded synthetic strategy for uniform polymer and carbon nanospheres with tunable sizes for high performance electrochemical energy storage, Chem. Commun. 49(2013) 3043-3045;(b) L. Jiang, J.W. Yan, L.X. Hao, et al., High rate performance activated carbons prepared from ginkgo shells for electrochemical supercapacitors, Carbon 56(2013) 146-154;(c) Y.S. Yun, S.Y. Cho, J.Y. Shim, et al., Microporous carbon nanoplates from regenerated silk proteins for supercapacitors, Adv. Mater. 25(2013) 1993-1998;(d) C.X. Zhang, D.H. Long, B.L. Xing, et al., The superior electrochemical performance of oxygen-rich activated carbons prepared from bituminous coal, Electrochem. Commun. 10(2008) 1809-1811. |
| [8] | Y.H. Zhao, M.X. Liu, L.H. Gan, et al., Ultramicroporous carbon nanoparticles for the high-performance electrical double-layer capacitor electrode, Energy Fuels 28(2014) 1561-1568. |
| [9] | (a) L.M. Dai, D.W. Chang, J.B. Baek, W. Lu, Carbon nanomaterials for advanced energy conversion and storage, Small 8(2012) 1130-1166;(b) H. Jiang, J. Ma, C.Z. Li, Mesoporous carbon incorporated metal oxidenanomaterials as supercapacitor electrodes, Adv. Mater. 24(2012) 4197-4202;(c) W. Li, F. Zhang, Y.Q. Dou, et al., A self-template strategy for the synthesis of mesoporous carbon nanofibers as advanced supercapacitor electrodes, Adv. Energy Mater. 1(2011) 382-386. |
| [10] | (a) B. Liu, H. Shioyama, T. Akita, Q. Xu, Metal-organic framework as a template for porous carbon synthesis, J. Am. Chem. Soc. 130(2008) 5390-5391;(b) H.L. Jiang, B. Liu, Y.Q. Lan, et al., From metal-organic framework to nanoporous carbon:toward a very high surface area and hydrogen uptake, J. Am. Chem. Soc. 133(2011) 11854-11857;(c) H. (Ⅰ)toi, H. Nishihara, T. Kogure, T. Kyotani, Three-dimensionally arrayed and mutually connected 1.2-nm nanopores for high-performance electric double layer capacitor, J. Am. Chem. Soc. 133(2011) 1165-1167;(d) B. Xu, S.S. Hou, H. Duan, et al., Ultramicroporous carbon as electrode material for supercapacitors, J. Power Sources 228(2013) 193-197;(e) M.X. Liu, J.S. Qian, Y.H. Zhao, et al., Core-shell ultramicroporous@microporous carbon nanospheres as advanced supercapacitor electrodes, J. Mater. Chem. A 3(2015) 11517-11526;(f) N.M. Pereira, P.M.V. Fernandes, C.M. Pereira, et al., Electrodeposition of zinc from choline chloride-ethylene glycol deep eutectic solvent:effect of the tartrate ion, J. Electrochem. Soc. 159(2012) D501-D506;(g) J. Geng, X.D. Jia, J.J. Zhu, Sonochemical selective synthesis of ZnO/CdS core/shell nanostructures and their optical properties, Cryst. Eng. Commun. 13(2011) 193-198;(h) G. Srinivas, V. Krungleviciute, Z.X. Guo, T. Yildirim, Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume, Energy Environ. Sci. 7(2014) 335-342. |
| [11] | K.S.W. Sing, D.H. Everett, R.A.W. Haul, et al., Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, Pure Appl. Chem. 57(1985) 603-619. |
| [12] | (a) W.J. Gao, Y. Wan, Y.Q. Dou, D.Y. Zhao, Synthesis of partially graphitic ordered mesoporous carbons with high surface areas, Adv. Eng. Mater. 1(2011) 115-123;(b) P. Simon, Y. Gogotsi,Materials for electrochemical capacitors, Nat.Mater. 7(2008) 845-854;(c) P. Yadav, A. Banerjee, S. Unni, et al., A 3D hexaporous carbon assembled from single-layer graphene as high performance supercapacitor, Chem-SusChem 5(2012) 2159-2164. |
| [13] | D.S. Yuan, J.X. Chen, S.X. Tan, N.N. Xia, Y.L. Liu, Worm-like mesoporous carbon synthesized from metal-organic coordination polymers for supercapacitors, Electrochem. Commun. 11(2009) 1191-1194. |
| [14] | S.M. Paek, E. Yoo, (Ⅰ). Honma, Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure, Nano Lett. 9(2009) 72-75. |
| [15] | (a) W. Xiong, M.X. Liu, L.H. Gan, et al., A novel synthesis of mesoporous carbon microspheres for supercapacitor electrodes, J. Power Sources 196(2011) 10461-10464;(b) Q. Wang, Q. Cao, X.Y. Wang, et al., A high-capacity carbon prepared from renewable chicken feather biopolymer for supercapacitors, J. Power Sources 225(2013) 101-107;(c) Q.H. Wang, L.F. Jiao, H.M. Du, Y.J. Wang, H.T. Yuan, Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors, J. Power Sources 245(2014) 101-106. |
| [16] | (a) C. Merlet, B. Rotenberg, P.A. Madden, et al., On the molecular origin of supercapacitance in nanoporous carbon electrodes, Nat. Mater. 11(2012) 306-310;(b) K. Xie, X.T. Qin, X.Z. Wang, Y.Y. Xia, Carbon nanocages as supercapacitor electrode materials, Adv. Mater. 24(2012) 347-352. |
| [17] | (a) H.J. Liu, J. Wang, C.X. Wang, et al., Ordered hierarchical mesoporous/microporous carbon derived from mesoporous titanium-carbide/carbon composites and its electrochemical performance in supercapacitor, Adv. Energy Mater. 1(2011) 1101-1108;(b) X.M. Ma, M.X. Liu, L.H. Gan, Y.H. Zhao, L.W. Chen, Synthesis of micro-and mesoporous carbon spheres for supercapacitor electrode, J. Solid State Electrochem. 17(2013) 2293-2301;(c) Y.K. Lv, L.H. Gan, M.X. Liu, et al., A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes, J. Power Sources 209(2012) 152-157. |
| [18] | Y.Y. Lv, F. Zhang, Y.Q. Dou, et al., A comprehensive study on KOH activation of ordered mesoporous carbons and their supercapacitor application, J. Mater. Chem. 22(2012) 93-99. |
| [19] | D. Feng, Y.Y. Lv, Z.X. Wu, et al., Free-standing mesoporous carbon thin films with highly ordered pore architectures for nanodevices, J. Am. Chem. Soc. 133(2011) 15148-15156. |
| [20] | D.W. Wang, F. Li, M. Liu, G.Q. Lu, H.M. Cheng, 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage, Angew. Chem. (Ⅰ)nt. Ed. 47(2008) 373-376. |
 2016, Vol.27
2016, Vol.27