b Department of Chemistry, Faculty of Science, Urmia University, 57159 Urmia, Iran
Compounds bearing benzoxazoles,benzothiazoles,or benzimidazoles have found applications as antibiotic [1],antifungal [2],antiviral [3],anticancer [4],antimicrobial [5],and antiparkinson [6] properties. The protocols usually followed for their synthesis involve condensation of orthoesters [7, 8, 9],nitriles [10],aldehydes [11, 12],carboxylic acids [13],acid chlorides [14],amides [15],and esters [16] with ortho-substituted aminoaromatics in the presence of different acids or catalysts,such as Pd-catalyzed oxidative cyclization [17],base assisted reaction of 1,1-dibromoethanes [18],different heteropolyacid catalysts [19],In(OTF)3 [20] and etc. All of these methods involve multistep synthesis,use of toxic reagents and solvent,long reaction times,low yields,using excess amounts of reagent and in the case of metal-containing reactions,polishing with metal scavengers is often required in order to remove metals from the reaction mixture. Therefore,there is a strong demand for a more effective,simple and environmentally friendly process for the synthesis of these heterocycles.
SBA-15 is an interesting mesoporous silica material having highly ordered nanopores and a large surface area,which is widely employed as absorbents,drug delivery materials and as solid materials for support of molecular catalysts in order to transform them into heterogeneous catalysts [21, 22, 23, 24].
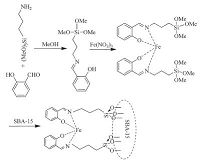
Iron(III) complexes of Schiff base ligands have been demonstrated to be efficient catalysts for a wide range of reactions [25]. In general,Schiff bases are prepared by the condensation of primary amines and aldehydes or ketones and are able to coordinate metals via nitrogen lone pair electrons [26]. The covalent anchoring of such complexes onto the SBA-15 is a practical way to produce heterogeneous catalysts (Scheme 1) with the associated advantages such as easy catalyst separation,possible catalyst recycling,and high activity and selectivity.
|
Download:
|
| Scheme 1.Preparation of Fe(III)-Schiff base/SBA-15. | |
Here we report the synthesis of benzimidazoles,benzoxazoles,and benzothiazoles derivatives by condensation of 1,2-phenylenediamine,ortho-aminophenol and ortho-aminothiophenol with various aromatic aldehydes using Fe(III)-Schiff base/SBA-15 as an efficient catalyst in water media.
2. ExperimentalTEM observation was performed with a Hitachi H-700 CTEM. FT-IR spectra were recorded on KBr pellets by a Jasco 4200 FT-IR spectrophotometer. X-ray diffraction experiments (XRD; Bruker D8ADVANCE with Ni-filtered Cu Kα radiation at 1.5406Å ) were recorded with a speed of 2 min-1 and a step of 0.05°. 1H NMR and 13C NMR spectra were recorded at room temperature on a Bruker AC 300,400 and 500 MHz spectrometers using CDCl3 or DMSO-d6 as the NMR solvents. 1H NMR spectra are referenced to tetramethylsilane (0.00 ppm) and 13C NMR spectra are referenced from the solvent central peak (for example,77.23 ppm for CDCl3). Chemical shifts are given in ppm. N2 adsorption/desorption isotherms were obtained at 77.35 K with a Quantachrome Autosorb-1 apparatus. Before measurements,the samples were outgassed at 120 ℃ for 12 h. The specific surface area and the pores size distributions were obtained from the desorption branch of the isotherms,respectively,using the Brunauer-Emmett-Teller (BET) method and Barrett-Joyner-Halenda (BJH) analyses. A Shimadzu AA-6300 flame atomic absorption spectrometer was used to obtain the concentration of metal ions. For this purpose,0.1 g of the catalyst was digested by HNO3 with stirring at room temperature for a week. Then the mixture was filtered and the solid was washed several times with water to gain a colourless filtrate solution for metal measurements. The concentration of Fe(III) in the immobilized SBA-15 was 0.016 mmol/g. Thermogravimetric analysis was carried out with a TGA/DTA Shimadzu-50 instrument equipped with a platinum pan. The samples were heated in air from 25 ℃ to 1000 ℃ with a heating rate of 10 ℃/min. The weight loss was recorded as a function of temperature. Melting points were recorded using a Buchi B540 melting point apparatus and are uncorrected.
Poly(ethylene glycol)-block-poly(propylene glycol)-blockpoly( ethylene glycol) (P123) triblock co-polymer,iron(III) nitrate,salicylaldehyde,(3-aminopropyl)trimethoxysilane (APTMS),tetraethyl orthosilicate (TEOS),HNO3,hydrochloric acid,ethylanediamine,commercial grade of 1,2-phenylenediamines,2- aminophenol,2-aminobenzenethiol,carbonyl compounds were purchased from Sigma-Aldrich,Merck and Acros chemical companies. All materials were used without further purification. The solvents used for the synthesis were of analytical grade and were used as received. Silica gel (Merck,grade 9385,230- 400 mesh,60Å ) for column chromatography was used as received. All other reagents were purchased from Merck and used as received unless otherwise noted. The course of the synthesis of heterocycles was followed by TLC on silica gel plates (Merck,silica gel 60 F254,ready to use),using n-hexane:ethyl acetate (4:1) as eluents.The eluent for column chromatography was the same as TLC eluent.
2.1. Anchoring of Fe(III)-Schiff-base complex on SBA-15SBA-15 was prepared by the procedure previously explained by Zhao et al. [27] we prepared Fe(III) Schiff-base complex by using procedure reported by Chisem et al. [28] Activated SBA-15 (1.5 g) was suspended in 20 mL methanol solution containing (0.38 g) Schiff base complex,and the mixture was stirred for 24 h. The solvent was removed using a rotary evaporator,and the resulting solid was dried at 80 ℃ overnight. The product was washed with MeOH and deionized until the washings were colourless to ensure that the non-covalently grafted complex and physisorbed metal species were removed. At last,the product was dried in an oven at 80 ℃ for 8 h (Scheme 1).
2.2. General procedure for the synthesis of heterocyclesA round-bottomed flask equipped with a magnet and condenser was charged with 1,2-phenylenediamine 1a or ortho-aminophenol 1b or ortho-aminothiophenol 1c (1.0 mmol),substituted benzaldehyde (1.0 mmol),water (2 mL) and catalyst Fe(III)-Schiff base/ SBA-15 catalyst (0.01 g,0.0014 mmol based on metal ions). The resulting mixture was stirred under reflux for the appropriate time,and the course of the reaction was monitored using TLC on silica gel. Finally,the crude mixture was purified by column chromatography or recrystallized in toluene to obtain the desired products. Spectral and physical data for heterocyclic products are reported in Supporting information [29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39].
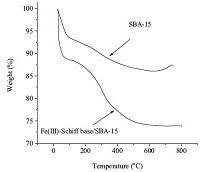
3. Results and discussionFig. 1 illustrates the TG profiles of the pure SBA-15 and Fe(III)- Schiff base supported on SBA-15. Pure siliceous SBA-15 shows a mass loss below 100 ℃ through the loss of physically adsorbed water. The thermogram of Fe(III)-Schiff-base anchored SBA-15 shows not only a mass loss due to dehydration,but also a more mass loss between 200 ℃ and 700 ℃,which could be attributed to loss of anchored Schiff-base ligand and organic spacer.
|
Download:
|
| Fig. 1.Thermogravimetric analysis results of: (a) SBA-15, (b) Fe(III)-Schiff base/ SBA-15. | |
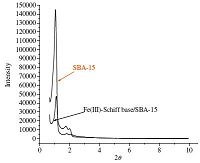
The quality and structural ordering of Fe(III)-Schiff base/SBA- 15 were measured by XRD. Fig. 2 represents the X-ray diffraction scheme of SBA-15 and Fe(III)-Schiff base/SBA-15. The XRD pattern of both SBA-15 and Fe(III)-Schiff base/SBA-15 represent reflections about (1 0 0),(1 1 0),and (2 0 0),which are associated with the typical two-dimensional hexagonal symmetry of the SBA-15 material. It shows that the ordered mesoporous structure of SBA-15 remains intact after anchoring of Fe(III)-Schiff base. The XRD peaks of Fe(III)-Schiff base/SBA-15 shift to lower angle than SBA-15,suggesting the development of unit cell due to the connection of the complex within SBA-15 [40].
|
Download:
|
| Fig. 2.XRD patterns of: (a) SBA-15 and (b) Fe(III)-Schiff base/SBA-15. | |
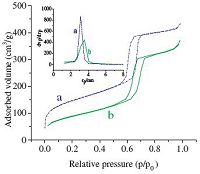
N2 adsorption/desorption isotherms of the SBA-15 and Fe(III)- Schiff base/SBA-15 samples are plotted in Fig. 3. Both materials exhibited the IV type isotherms with H1 hysteresis loop,according to IUPAC classification,characteristic of mesoporous materials. [41] As can be seen in Table 1,SBA-15 has a specific surface area 539 m2/g and pore volume of 1.1 cm3/g,while Fe(III)-Schiff base/ SBA-15 has a specific surface area 472 m2/g and pore volume 0.85 cm3/g. Thereby,the presence of Fe(III)-Schiff base anchored on SBA-15 surface induces remarkable decrease in the surface area and in mesopore volume.
|
Download:
|
| Fig. 3.Nitrogen adsorption/desorption isotherms incorporated with pore size distributions at inset of: (a) SBA-15 and (b) Fe(III)-Schiff base/SBA-15. | |
|
|
Table 1 Textural properties of the SBA-15 and Fe(III)-Schiff base/SBA-15. |
Atomic absorption spectroscopy was carried out to determine the concentration of iron(III) in the catalyst which was 0.14 mmol/ g. The elemental analysis of supported catalyst showed that the nitrogen/iron molar ratio was 1.7,which is in good agreement with the expected value of 2. As can be seen in Scheme 1,this result confirms formation of the Fe(III)-Schiff base complex with a N2O2 ligand environment [42, 43, 44].
Fig. 4 shows TEM image of Fe(III)-Schiff base/SBA-15. TEM image of Fe(III)-Schiff base/SBA-15 confirms the retaining of 2Dhexagonal array of uniform linear channels after the complex anchoring.

|
Download:
|
| Fig. 4.TEM image of Fe(III)-Schiff base/SBA-15. | |
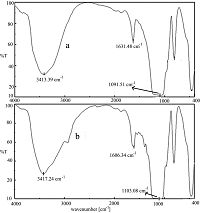
Fig. 5 demonstrates the FT-IR spectra of SBA-15 and Fe(III)- Schiff base/SBA-15. Fe(III)-Schiff base/SBA-15 shows not only the silica framework band such as the peaks between 1000 and 1200 cm-1,which is due to stretch vibrations of (Si-O-Si) bonds,but also it shows the Schiff base complex bands such as C=N stretching bands at about 1608 cm-1 and a peak at about 2960 cm-1 due to aliphatic CH2 stretching bonds.
|
Download:
|
| Fig. 5.FT-IR spectra of (a) SBA-15 and (b) Fe(III)-Schiff base/SBA-15 catalytic synthesis of heterocycles. | |
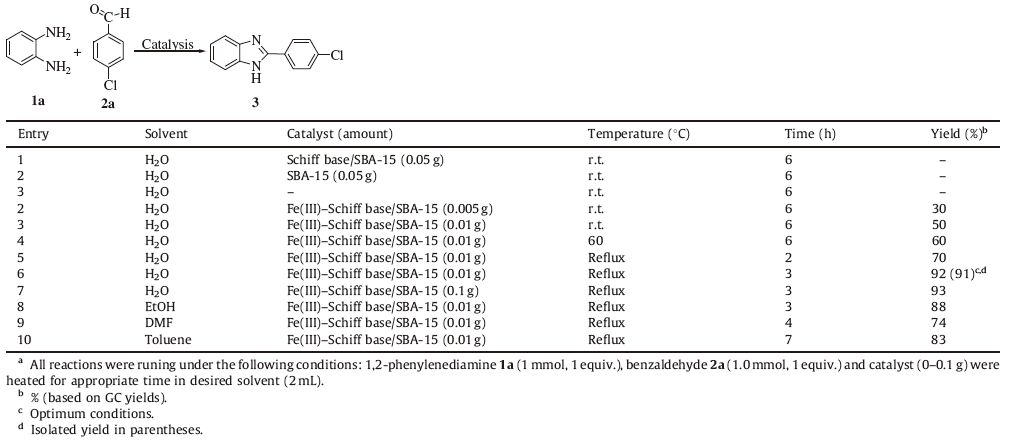
After the survey of the structure and morphology of the prepared Fe(III)-Schiff base functionalized SBA-15,we intended to test its efficiency as heterogeneous catalysts for the preparation of benzoxazoles,benzothiazoles,and benzimidazoles in water. At first,1,2-phenylenediamine 1a and para-chlorobenzaldehyde 2a were selected as the model substrates to investigate the best reaction conditions (Table 2). Then several reaction conditions were tried to accomplish this reaction and the efficiency of the reaction was found to be influenced by the quantity of the catalyst,temperature and solvent. In the absence of catalyst or by using either Schiff base/SBA-15 or SBA-15 as catalyst,we gave no yield of product at room temperature in water (Table 2,entry 1). The best yield was obtained at reflux temperature and in the presence of 0.01 g (0.0014 mmol based on Fe ions) of catalyst (Table 2,entry 6). Although using of water,alcohols and toluene as a solvent gave reasonable yields (Table 2,entries 6,8 and 10),the opportunity of applying a cheap solvent such as water is an environmentally significant benefit of the procedure.
|
|
Table 2 Screening of the reaction conditions for the reaction of 1,2-phenylenediamine 1a and para-chlorobenzaldehyde 2a.a |
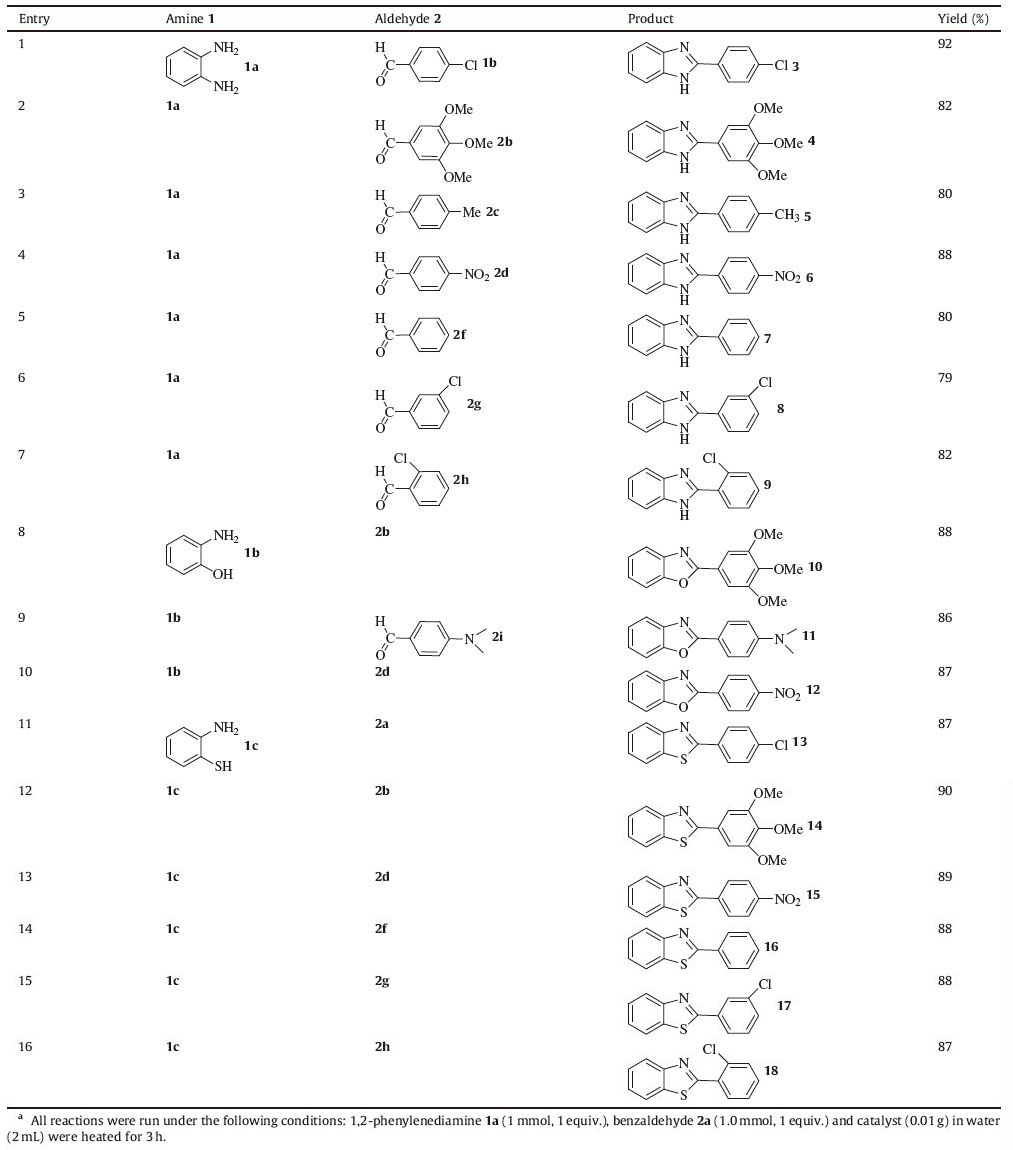
The generality of the procedure was evaluated by reactions of different o-aminophenol,o-aminothiophenol,and o-phenylenediamine with different aldehydes (Table 3). As shown in Table 3,good to excellent yields were obtained for most of these reactions. The reactivity of o-phenylenediamine with aldehyde derivatives appear to be controlled by electronic effects. For example,1,2- phenylenediamine 1a and aldehydes bearing electron withdrawing group at the para-position of aromatic rings gave higher yields (Table 3,entries 1,4) due to the increase in electrophilicity of the carbonyl carbon of the aldehydes. Reactivities of ortho-aminothiophenol 1c and ortho-aminophenol 1b with both electron-rich and electron-deficient aldehydes are good and affording benzothiazoles and benzoxazoles in high yields (Table 3,entries 8-16).
|
|
Table 3 Synthesis of desired heterocyclic derivatives catalyzed by Fe(III)-Schiff base/SBA-15a. |
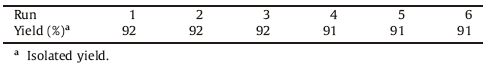
In continue,the reusability of the catalyst was examined for the model reaction (compound 3). For this,the catalyst was separated and reused after washing with hot ethanol and drying at 80 ℃ for 60 min. The recovered catalyst was employed for further reactions and Fe(III)-Schiff base/SBA-15 turnover number was found to be six (Table 4).
|
|
Table 4 Reuse of the catalyst for the synthesis of 2-(4-chlorophenyl)benzimidazole 3. |
A new synthetic method for the synthesis of benzoxazoles,benzothiazoles and benzimidazoles using Fe(III)-Schiff base/ SBA-15 as a heterogeneous nanocatalyst in water have been developed. The use of Fe(III)-Schiff base/SBA-15 as a reusable efficient heterogeneous catalyst,water as a green solvent,high yields of products and nontoxic nature of the catalyst makes this protocol practical,environment-friendly and economically attractive.
AcknowledgmentThe authors wish to thank Payame Noor University for its financial support of this study.
Appendix A. Supplementary dataSupplementarymaterial related to this article canbe found,inthe online version,at http://dx.doi.org/10.1016/j.cclet.2015.10.011.
| [1] | D.A. Evans, C.E. Sacks, W.A. Kleschick, T.R. Taber, Polyether antibiotics synthesis. Total synthesis and absolute configuration of the ionophore A-23187, J. Am. Chem. Soc. 101 (1979) 6789-6791. |
| [2] | M.J. Yamato, Study on the development of biological-active compounds after the model of natural products, Pharm. Soc. Jpn. 112 (1992) 81-99. |
| [3] | X. Song, B.S. Vig, P.L. Lorenzi, et al., Amino acid ester prodrugs of the antiviral agent 2-bromo-5,6-dichloro-1-(β-D-ribofuranosyl) benzimidazole (BDCRB) as potential substrates of hPEPT1 transporter, J. Med. Chem. 48 (2005) 1274-1277. |
| [4] | D. Kumar, M.R. Jacob, M.B. Reynolds, S.M. Kerwin, Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1, Bioorg. Med. Chem. 10 (2002) 3997-4004. |
| [5] | O.I. Yildiz, I. Yalcin, E. Aki-Sener, N. Ucarturk, Synthesis and structure-activity relationships of new antimicrobial active multisubstituted benzazole derivatives, Eur. J. Med. Chem. 39 (2004) 291-298. |
| [6] | A. Benazzou, T. Boraund, P. Dubedat, J.M. Boireau, C. Stutzmann, Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study, Eur. J. Pharmcol. 284 (1995) 299-307. |
| [7] | D. Villemin, M. Hammadi, B. Martin, Clay catalysis: condensation of orthoesters with o-substituted aminoaromatics into heterocycles, Synth. Commun. 26 (1996) 2895-2899. |
| [8] | M. Doise, F. Dennin, D. Blondeau, H. Sliwa, Synthesis of novel heterocycles: oxazolo[4,5-b] pyridines and oxazolo[4,5-d] pyrimidines, Tetrahedron Lett. 31 (1990) 1155-1156. |
| [9] | G.L. Jenkins, A.M. Knevel, C.S. Davis, A new synthesis of the benzothiazole and benzoxazole rings, J. Org. Chem. 26 (1961) 274-276. |
| [10] | D.W. Hein, R.J. Alheim, J.J. Leavitt, The use of polyphosphoric acid in the synthesis of 2-aryl-and 2-alkyl-substituted benzimidazoles, benzoxazoles and benzothiazoles, J. Am. Chem. Soc. 79 (1957) 427-429. |
| [11] | P. Salehi, M. Dabiri, M.A. Zolfigol, S. Otokesh, M. Baghbanzadeh, Selective synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles in water at ambient temperature, Tetrahedron Lett. 47 (2006) 2557-2560. |
| [12] | M. Mohammadi, G.R. Bardajee, N.N. Pesyan, A novel method for the synthesis of benzothiazole heterocycles catalyzed by a copper-DiAmSar complex loaded on SBA-15 in aqueous media, RSC Adv. 4 (2014) 62888-62894. |
| [13] | Y.H. So, J.P. Heeschen, Mechanism of polyphosphoric acid and phosphorus pentoxide-methanesulfonic acid as synthetic reagents for benzoxazole formation, J. Org. Chem. 62 (1997) 3552-3561. |
| [14] | R.N. Nadaf, S.A. Siddiqui, T. Daniel, R.J. Lahoti, K.V. Srinivasan, Room temperature ionic liquid promoted regioselective synthesis of 2-aryl benzimidazoles, benzoxazoles and benzthiazoles under ambient conditions, J. Mol. Catal., A: Chem. 214 (2004) 155-159. |
| [15] | M. Terashima, M.A. Ishii, A facile synthesis of 2-substituted benzoxazoles, Synthesis (6) (1982) 484-485. |
| [16] | A.K. Chakraborti, S. Rudrawar, G. Kaur, L. Sharma, An efficient conversion of phenolic esters to benzothiazoles under mild and virtually neutral conditions, Synlett 9 (2004) 1533-1536. |
| [17] | Y. Pang, W. Hua, Efficient synthesis of 2-(2'-hydroxyphenyl)benzoxazole by palladium(Ⅱ)-catalyzed oxidative cyclization, Tetrahedron Lett. 50 (2009) 6680-6683. |
| [18] | W. Shen, T. Kohn, Z. Fu, et al., Synthesis of benzimidazoles from 1,1-dibromoethenes, Tetrahedron Lett. 49 (2008) 7284-7286. |
| [19] | M.M. Heravi, S. Sadjadi, H.A. Oskoose, R.H. Shoar, Heteropoly acids as heterogeneous and recyclable catalysts for the synthesis of benzimidazoles, Catal. Commun. 9 (2008) 504-507. |
| [20] | R. Trivedi, S.K. De, R.A. Gibbs, A convenient one-pot synthesis of 2-substituted benzimidazoles, J. Mol. Catal., A: Chem. 245 (2006) 8-11. |
| [21] | G.R. Bardajee, R. Malakooti, I. Abtin, H. Atashin, Palladium Schiff-base complex loaded SBA-15 as a novel nanocatalyst for the synthesis of 2,3-disubstituted quinoxalines and pyridopyrazine derivatives, Microporous Mesoporous Mater. 169 (2013) 67-74. |
| [22] | G.R. Bardajee, R. Malakooti, F. Jami, Z. Parsaei, H. Atashin, Covalent anchoring of copper-Schiff base complex into SBA-15 as a heterogeneous catalyst for the synthesis of pyridopyrazine and quinoxaline derivatives, Catal. Commun. 27 (2012) 49-53. |
| [23] | M. Shakeri, R.J.M. Klein Gebbink, P.E. de Jongh, K.P. de Jong, Control and assessment of plugging of mesopores in SBA-15 materials, Microporous Mesoporous Mater. 170 (2013) 340-345. |
| [24] | J. Liu, Y. Liu, W. Yang, et al., A novel and simple strategy for the direct synthesis bimetallic mesoporous materials Zr-La-SBA-15, Mater. Lett. 128 (2014) 15-18. |
| [25] | K.C. Gupta, A.K. Sutar, Catalytic activities of Schiff base transition metal complexes, Coord. Chem. Rev. 252 (2008) 1420-1450. |
| [26] | P.G. Cozzi, Metal-Salen Schiff base complexes in catalysis: practical aspects, Chem. Soc. Rev. 33 (2004) 410-421. |
| [27] | D. Zhao, J. Feng, Q. Huo, et al., Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores, Science 279 (1998) 548-552. |
| [28] | I.C. Chisem, J. Rafelt, M.T. Shieh, et al., Catalytic oxidation of alkyl aromatics using a novel silica supported Schiff base complex, Chem. Commun. 18 (1998) 1949-1950. |
| [29] | B.A. Abdelkrim, B. Khalid, S. Mohamed, Synthèse chimiosélective des benzimidazoles sur silice traitée par le chlorure du thionyle, Tetrahedron Lett. 44 (2003) 5935-5937. |
| [30] | H. Goker, C. Ku, D.W. Boykin, S. Yildiz, N. Altanar, Synthesis of some new 2-substituted-phenyl-1H-benzimidazole-5-carbonitriles and their potent activity against Candida species, Bioorg. Med. Chem. 10 (2002) 2589-2596. |
| [31] | K. Khosravi, S. Kazemi, Synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles in both room temperature and microwave condition catalyzed by hexamethylenetetramine-bromine complex, Chin. Chem. Lett. 23 (2012) 61-64. |
| [32] | S.B. Sapkal, K.F. Shelke, S.S. Sonar, B.B. Shingate, M.S. Shingare, Acidic ionic liquid catalyzed environmentally friendly synthesis of benzimidazole derivatives, Bull. Catal. Soc. India 2 (2009) 78-83. |
| [33] | D.V. Ramana, E. Kantharaj, Synthesis of 2-substituted benzoxazoles and benzimidazoles based on mass spectral ortho interactions, J. Chem. Soc., Perkin Trans. 2 (1995) 1497-1501. |
| [34] | R.S. Pottorf, N.K. Chadha, M. Katkevics, et al., Parallel synthesis of benzoxazoles via microwave-assisted dielectric heating, Tetrahedron Lett. 44 (2003) 175-178. |
| [35] | R.S. Varma, R.K. Saini, O. Prakash, Hypervalent iodine oxidation of phenolic Schiff's bases: synthesis of 2-arylbenzoxazoles, Tetrahedron Lett. 38 (1997) 2621-2622. |
| [36] | M.M. Heravi, N. Abdolhosseini, H.A. Oskooie, Re-gioselective and high-yielding bromination of aromatic compounds using hexamethylenetetramine-bromine, Tetrahedron Lett. 46 (2005) 8959-8963. |
| [37] | S.V. Nalage, S.V. Bhosale, D.S. Bhosale, W.N. Jadhav, P2O5 mediated rapid condensation of 2-aminothiophenol with aromatic aldehydes at ambient temperature, Chin. Chem. Lett. 21 (2010) 790-793. |
| [38] | Y. Li, Y.L. Wang, J.Y. Wang, A simple iodine-promoted synthesis of 2-substituted benzothiazoles by condensation of aldehydes with 2-aminothiophenol, Chem. Lett. 35 (2006) 460-461. |
| [39] | M. Okimoto, T. Yoshida, M. Hoshi, et al., Electrooxidative cyclization of benzylideneaminothiophenols to the corresponding 2-arylbenzothiazoles, Heterocycles 75 (2008) 35-42. |
| [40] | F.M. Masteri, F. Farzaneh, M. Ghandi, Synthesis and characterization of molybdenum complexes with bidentate Schiff base ligands within nanoreactors of MCM-41 as epoxidation catalysts, J. Mol. Catal., A: Chem. 248 (2006) 53-60. |
| [41] | W.H. Zhang, X.B. Lu, J.H. Xiu, et al., Synthesis and characterization of bifunctionalized ordered mesoporous materials, Adv. Funct. Mater. 14 (2004) 544-552. |
| [42] | S. Jana, B. Dutta, R. Bera, S. Koner, Anchoring of copper complex in MCM-41 matrix: a highly efficient catalyst for epoxidation of olefins by tert-BuOOH, Langmuir 23 (2007) 2492-2496. |
| [43] | U.G. Singh, R.T. Williams, K.R. Hallam, G.C. Allen, Exploring the distribution of copper-Schiff base complex covalently anchored onto the surface of mesoporous MCM 41 silica, J. Solid State Chem. 178 (2005) 3405-3413. |
| [44] | S. Singha, K.M. Parida, A.C. Dash, Fe(Ⅲ)-salim anchored MCM-41: synthesis, characterization and catalytic activity towards liquid phase cyclohexane oxidation, J. Porous Mater. 18 (2011) 707-714. |
 2016, Vol.27
2016, Vol.27