b School of Materials and Chemical Engineering, Anhui Jianzhu University, Hefei 230601, China;
c Guangdong Tianan New Material Co., Ltd., Foshan 528000, China
Poly(ethylene terephthalate) (PET) is a widely-used plastic due to its outstanding properties,such as high strength,resistance to stretch and shrinkage [1]. Recently,the modification of PET by grafting active groups to chelate heavy metal ions (Cu2+,Fe3+,Co2+,Ni2+,etc.) from waste water has attracted much attention [2, 3, 4]. For example,the grafting polymerization of methacrylic acid can be induced on PET fiber under the radiation of ultraviolet [5]. Rahman and his coworkers used g-ray to induce the grafting polymerization of acrylic acid on PET film,and investigated the selective adsorption behavior of Cu2+ in the aqueous solution at the presence of Co2+ and Ni2+ [6]. Cyano group (-CN) and its derivative groups (e.g. amidoxime group) possess good chelation ability to metal ions [7]. However,it is difficult to introduce cyano groups on PET chains through the grafting polymerization of acrylonitrile (AN) due to the excellent chemical stability of PET. In our previous work,PETg- PAN composite films were fabricated by g-ray radiation-induced graft polymerization of AN on PET film in an aqueous solution after the PET film was firstly grafted with poly(acrylic acid) (PAA) [8]. Unfortunately,the gas or water permeability of the PET-g-PAN composite film will become much worse because the polar -CN side groups on PET chains produce strong intermolecular interaction so as to reduce the free volume for the diffusion of gas or water molecules in the grafted PAN layer. Therefore,if PAN can be grafted on PET microspheres,the -CN groups can play their roles to the maximum degree since the modified PET microspheres can be easily dispersed into the aqueous solution,and have enough specific surface area compared with the macroscopic film material.
However,there are few reports on the preparation of PET microspheres due to the limitation of the synthesis method and the poor solubility in most solvents of PET. Conventionally,polymer microspheres are produced through heterogeneous polymerization (such as miniemulsion or emulsion polymerization). However,PET is synthesized via transesterification at a temperature over 150 ℃ or bulk polycondensation reaction of terephthalic acid and ethylene glycol at a temperature over 200 ℃ [9]. Therefore,it is impossible to form PET microspheres directly during the polymerization process. Burton and O’Farrell had proposed a method to prepare polymer microspheres via the evaporation of the solvent from emulsion droplets in 1977 [10]. This method had been widely investigated and applied mainly in drugs encapsulation and controlled release in pharmaceutical science [11, 12]. In this process,polymer is firstly dissolved in a good solvent. The solution is subsequently used as the oil phase to prepare an emulsion. Then the solvent in the emulsion droplet is evaporated by heating or under vacuum [13],and the polymer chains solidify into microspheres simultaneously. Microspheres with various morphologies can be produced via this method,such as hollow microspheres [14, 15] and Janus microspheres [16, 17, 18]. Nevertheless,there are no reports on the formation of PET microspheres by this method since most of the good solvents for PET are polar and nonvolatile,which makes it difficult to form PET emulsion and remove the solvent.
In this work,we herein firstly explored a proper solvent for PET to form a stable emulsion,and then let PET precipitate in ethanol to form a spherical microsphere. Subsequently,PAN microspheres were formed on the surface of the prepared PET microspheres by γ-ray radiation-induced polymerization of AN to form a raspberrylike PET/PAN composite microspheres. This work provides a new way to prepare micron-sized PET microspheres,and further expands the functionalized modification method of PET and its application area.
2. ExperimentalAnalytical reagents including phenol,1,1,2,2-tetrachloroethane (TCE),sodium dodecyl sulfate (SDS),ferrous sulfate hydrate (FeSO4·7H2O),and ethanol were purchased from Sinopharm Chemical Reagent Co. Ltd.,and used as received. Chemical-grade acrylic acid (AA; Shanghai Chemical Reagents Co.) was purified by vacuum distillation,and stored at -20 ℃ before use. Acrylonitrile (AN) bought from Shanghai Lingfeng Chemical Reagent Co. was purified by passing through a column packed with basic aluminum oxide (Sinopharm Chemical Reagent Co.). PET granules (CB 651) were produced by Shanghai BangKai Co.,China,and provided by Changchun Institute of Applied Chemistry,Chinese Academy of Sciences. Deionized water was used in all experiments.
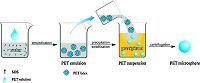
2.1. Preparation of PET microspheresThe preparation process of PET microspheres is illustrated in Scheme 1. First,2 g of PET granules were added into 98 g of the solvent. The system was stirred and heated to 95 ℃ in an oil bath for 30 min,and then cooled down to room temperature slowly. The PET solution was then added dropwise into the aqueous solution of SDS (2 wt%) under ultrasonic to form a stable emulsion. The obtained PET emulsion was then added into ethanol to let PET precipitate from the emulsion to form a suspension. Subsequently,the produced PET microspheres were separated from the suspension by centrifugation,washed with ethanol to remove the residual phenol and TCE,and finally dried at 60 ℃ for 12 h.
|
Download:
|
| Scheme 1.The schematic of the preparation process for PET microspheres. | |
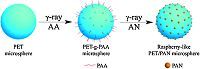
A two-step radiation-induced grafting polymerization was adopted to form raspberry-like PET/PAN microspheres,as illustrated in Scheme 2. FeSO4·7H2O (0.5 g),water (32 g),and acrylic acid (17.5 g) were mixed into a transparent solution under magnetically stirring. Here,Fe2+ ions served as the inhibitor of the homopolymerization of monomers in order to enhance the probability of the grafting polymerization [19]. The as-prepared PET microspheres (0.5 g) were dispersed into the above solution. After being bubbled nitrogen for 5 min,the system was sealed immediately,and placed into a 60Co γ-ray source (located in University of Science and Technology of China,= kCi). The absorbed dose was controlled as 28 kGy at a dose rate of 22 Gy min-1. The treated PET microspheres,labeled as PET-g- PAA,were collected by centrifugation,and ultrasonically washed with water.
|
Download:
|
| Scheme 2.The synthesis of raspberry-like PET microspheres via a two-step radiation-induced grafting polymerization process. | |
Next,0.1 g of the as-prepared PET-g-PAA microspheres was dispersed into 100 mL of water containing 0.05 g of AN under magnetic stirring. After being bubbled with nitrogen for 10 min,the dispersion was sealed immediately,and then exposed to 60Co γ-ray radiation at a dose rate of 40 Gy min-1 and an absorbed dose of 58 kGy. After the irradiation,the obtained microspheres were collected by centrifugation,and ultrasonically washed with ethanol.
2.3. CharacterizationThe morphology of the prepared microspheres was observed by TEM (Hitachi H-7650,100 kV) and SEM (JEOL JSM6700,5.0 kV). The samples were prepared by dispersing one drop of the ethanol dispersion of the product on copper grids and then air drying. Prior to SEM imaging,a thin layer of gold was spray-coated onto the sample to avoid the surface charging in the electron beam. The particle size and size distribution of the microspheres were obtained by measuring at least 100 microspheres with Adobe Photoshop CS5,and calculated according to the following equations:

The size and size distribution of PET microspheres were also measured by Nanosight LM10 Nanoparticles Analysis System (Salisbury,UK) with the NTA 2.2 software. The PET microspheres were dispersed in ethanol by ultrasonification before measurement.
The surface wettability of the prepared PET microspheres or raspberry-like microspheres was evaluated by static water contact angle (CA). The PET microspheres or raspberry-like microspheres were dispersed ultrasonically in ethanol to form a suspension. A piece of slide glass was immersed into the suspension for 2 min. After the glass slide was taken out and dried in an air-dry oven at = 55℃,a white particulate film was formed. The CA of the film was measured by Contact Angle Meter SL200B (Solon Tech. Co.,Ltd.). A droplet of water (2 μL) was dropped onto the film. The water contact angles were measured on five different sites for each sample,and recorded by the software (CAST 2.0) in the computer. The mean value was taken as the final result. Double-distilled water with a surface tension of 72 mN m-1 was used in this measurement.
3. Results and discussion3.1. Preparation and morphology control of PET microspheres
The precipitation from emulsion maybe the only practical way to prepare PET microspheres due to the limitation of the polymerization method and poor solubility of PET. However,to prepare a stable PET emulsion,the selection of a proper solvent is crucial. First,the solvent should have an enough good solubility for PET. Second,the solvent should be insoluble in water,but soluble in the precipitant to ensure the successful formation of PET microspheres from the emulsion in the precipitant (e.g. ethanol). Hexafluoroacetone and trifluoroacetic acid are good solvents for PET. But both of them are soluble in water. Phenol only slightly dissolves in water (8.3 g/100 g water,20 ℃),and it is found to be a good solvent for PET when it is heated up to 90 ℃,as shown in Fig. 1A (inset). However,when it is cooled to room temperature,phenol will crystallize because it has a relative high melting temperature (40.6 ℃) so that the solution will change into solid,as shown in Fig. 1A. Obviously,the frozen phenol solution of PET cannot be dispersed in water to form the emulsion at room temperature.

|
Download:
|
| Fig. 1.The solubility of PET in different solvents after the solution was cooled down from 95 ℃ to room temperature. (A) phenol (The inset is at 95 ℃); (B) TCE; (C) phenol/TCE mixed solvent (1/2, w/w). | |
If the homogeneous hot phenol solution of PET was directly mixed with water containing 2 wt% of SDS as the emulsifier at room temperature under ultrasonification,a stable PET emulsion still cannot be formed. On the contrary,PET will directly precipitate from water in the form of microspheres since phenol can be miscible with water at high temperature (above 65 ℃),as shown in Fig. 2A. The formation of PET microspheres should be attributed to the existence of the emulsifier because at the initial stage of the mixing,the emulsifiers will be adsorbed on the surface between the liquid PET phase and water to lower the surface tension and form the spherical liquid PET droplets. Then the liquid PET droplets are in-situ solidified due to the dissolution of phenol in water. However,the solidification process will be finished quickly in this condition due to the crystallization and low solubility of phenol in water at room temperature,which results in the uncontrollable size and size distribution,as well as the rough surface of the produced PET microspheres. Obviously,phenol is not the best solvent for the preparation of PET microspheres.

|
Download:
|
| Fig. 2.The SEM images of PET microspheres prepared from different conditions: (A) adding the hot phenol solution of PET into the aqueous solution of SDS (2 wt%); (B) adding the PET emulsion in ethanol. The PET emulsion was prepared by mixing the phenol/TCE (1/2, w/w) solution of PET with the aqueous solution of SDS (2 wt%). | |
Tetrachloroethane (TCE) is a polar solvent,which is theoretically favorable to dissolve the polar PET. But actually,PET even cannot be swollen in TCE,as shown in Fig. 1B. However,phenol can be dissolved in TCE. At the same time,TCE is insoluble in water,but soluble in ethanol. Thus,the mixture of phenol and TCE maybe a good solvent for PET,and be suitable for the preparation of PET emulsion. Fig. 1C shows that PET can also be rapidly dissolved in the mixture of phenol and TCE (phenol/TCE) with a weight ratio of 1/2 at 95 ℃. When the solution is cooled to room temperature,it is still a clear and stable PET solution without any crystallization of phenol. Thus a stable o/w emulsion is formed when the above PET solution is homogenized in the aqueous solution of SDS since both of TCE and phenol have a bad solubility in water at room temperature. The oil droplets are composed of the PET solution. What’s more,the size of the oil droplets can be adjustable by the concentration of the emulsifier,just like a common emulsion system. Therefore,when the prepared PET emulsion is added into a large amount of precipitant,e.g. ethanol,under stirring,the solvent (phenol/TCE) in the oil phase dissolves in ethanol,and PET in the oil phase will precipitate with a shape of microsphere,as shown in Fig. 2B. Clearly,submicron-sized PET microspheres with smooth surface can be successfully fabricated from the emulsionprecipitation method when the weight ratio of precipitant to emulsion (Wethanol/emulsion ) is 8. At the same time,the size distribution of PET microspheres seems to be much narrower. Evidently,the precipitation speed of polymer will depend on the Wethanol/emulsion ,which will further influence on the size of the polymer microspheres. Therefore,the influence of the Wethanol/ emulsion on the size and size distribution of the PET microspheres has been investigated by laser particle size analyzer,as shown in Fig. 3. It is clearly seen that the mean size of the precipitated PET microspheres decreases remarkably from 450 nm to 212 nm when the Wethanol/emulsion increases from 3 to 8. The result can be explained by the fact that the larger the Wethanol/emulsion ,the faster the precipitation of PET microspheres and the lower the concentration of the particles,which can avoid as far as possible the adsorption of newly added droplets on the solidified PET particle to form a larger one since the PET emulsion was dropwise added into ethanol. As a result,the size of PET microsphere becomes smaller with the increase of the Wethanol/emulsion . The size distribution also reflects that the coalescence of the droplets becomes serious with the decrease of the Wethanol/emulsion since the difference between the corresponding mean size and D50 or D90 becomes larger.

|
Download:
|
| Fig. 3.The size distribution of PET microspheres precipitated from ethanol under different Wethanol/emulsion. | |
It is noted here that the concentration of PET in the solvent of phenol/TCE should theoretically affect the morphology of PET microspheres. But in fact,if the concentration of PET in the solvent of phenol/TCE (1/2 w/w) is above 2 wt%,PET cannot be dissolved well. If the concentration of PET is lower than 1 wt%,the morphology of PET microspheres cannot be controlled well. The optimum concentration for the preparation of PET microspheres is between 1 wt% and 2 wt%,in which the morphology of the prepared PET microspheres has little difference.
3.2. Synthesis and characterization of PET/PAN raspberry-likemicrospheres In previous work,we found that PAN can be grafted on poly(acrylic acid)-modified PET film by γ-ray radiation technique [8]. Here,we followed the same method to graft AN to PET. As shown in Fig. 4A,the as-prepared PET microspheres were directly irradiated in the aqueous solution of AN,and the grafting of PAN on the PET microspheres cannot take place. This should be attributed to great difference between the radiation chemical yield (also known as G value,stands for the number of produced or destroyed species for each 100 eV absorbed by a substance) of PET and AN. As for the radiation grafting,if the G value of polymer is much lower than that of the monomer,the homopolymerization of monomer prevails over the grafting polymerization. Here,the GAN(R·) is 5.6,much higher than GPET(R·) (0.02) [20],which means it’s difficult to grafting PAN to PET directly. Therefore,a two-step radiation grafting process should be employed,i.e.,PAA is firstly grafted on PET microspheres,followed by the grafting of PAN because the G value of PAA is 3.6 [21],close to that of AN. Fig. 4B shows that PET microspheres have no obvious change in the size after being grafted with PAA,as well as the static water contact angle (CA) (see the insets of Fig. 4A and B). However,Fig. 4C clearly shows that PAN particles with a size of about 100 nm have been grafted on the PET-g-PAA microspheres to form a raspberry-like morphology. The grafting of PAN greatly changed the surface hydrophilicity of the microspheres,as seen from the inset picture of Fig. 4C. The CA of the particulate film consisting of raspberry-like PET/PAN microspheres is 778,higher than that of the film consisting of pure PET microspheres (45 ℃). The increase of the CA should be attributed to the hydrophobicity of PAN and hierarchical surface structure caused by the raspberry-like morphology of the microspheres.

|
Download:
|
| Fig. 4.The SEM images of PET microspheres after being irradiated by g-ray in different monomer solutions: (A) in the aqueous solution of AN; (B) in the aqueous solution of AA; and (C) successively irradiated in the aqueous solution of AA and AN. The insets are the CAs of the films consisting of the corresponding microspheres. | |
In this work,a facile emulsion-precipitant process was first reported to form submicron-sized PET microspheres from the commercial PET resins. The PET emulsion was formed by mixing water and a PET solution with the mixture of phenol and 1,1,2,2- tetrachloroethane (phenol/TCE) as the solvent at the presence of emulsifier SDS. After the PET emulsion was dropped into the precipitant (ethanol),PET microspheres can be successfully prepared. The size and size distribution of the PET microspheres can be adjusted by the weight ratio of ethanol to the PET emulsion (Wethanol/emulsion ). The higher the Wethanol/emulsion ,the smaller the size of PET microspheres and the narrower the size distribution. Based on the successful preparation of PET microspheres,raspberry-like PET/PAN microspheres can be synthesized through a successively g-ray-radiation-induced grafting polymerization of AA and AN on the surface of the PET microspheres. The size of the grafted PAN microspheres is about 100 nm. This work provides a new way to fabricate submicron-sized PET microspheres,and further expands the functionalized modification process of PET and its application fields.
5. AcknowledgmentsThe authors gratefully acknowledge the National Natural Science Foundation of China (Nos. 51573174,51473172,51173175,51073146 and 51103143),Foshan Scientific and Technological Innovation Team Project (No. 2013IT100041),and the Fundamental Research Funds for the Central Universities (Nos. WK2060200012 and WK3452016020301).
| [1] | L.Z. Xie, Y.Y. Xie, Q.H. Wu, et al., Effect of poly(acrylic acid)-modified poly(ethylene terephthalate) on improving the integrated mechanical properties of poly(ethylene terephthalate)/elastomer blend, Ind. Eng. Chem. Res. 54 (2015) 4748-4755. |
| [2] | D.R. Johnson, F. Tian, M.J. Roman, E.A. Decker, J.M. Goddard, Development of ironchelating poly(ethylene terephthalate) packaging for inhibiting lipid oxidation in oil-in-water emulsions, J. Agric. Food Chem. 63 (2015) 5055-5060. |
| [3] | J.N. Wang, L. Xu, C. Cheng, Y. Meng, A.M. Li, Preparation of new chelating fiber with waste pet as adsorbent for fast removal of Cu2+ and Ni2+ from water: kinetic and equilibrium adsorption studies, Chem. Eng. J. 193-194 (2012) 31-38. |
| [4] | Y. Meng, J.N. Wang, L. Xu, A.M. Li, Fast removal of Pb2+ from water using new chelating fiber modified with acylamino and amino groups, Chin. Chem. Lett. 23 (2012) 496-499. |
| [5] | L. Xu, J.N. Wang, Y. Meng, A.M. Li, Fast removal of heavy metal ions and phytic acids from water using new modified chelating fiber, Chin. Chem. Lett. 23 (2012) 105-108. |
| [6] | N. Rahman, N. Sato, S. Yoshioka, et al., Selective Cu(Ⅱ) adsorption from aqueous solutions including Cu(Ⅱ), Co(Ⅱ), and Ni(Ⅱ) by modified acrylic acid grafted PET film, ISRN Polym. Sci. (2013) 1-9, Article ID:536314. |
| [7] | S.H. Othman, M.A. Sohsah, M.M. Ghoneim, The effects of hazardous ions adsorption on the morphological and chemical properties of reactive cloth filter, Radiat. Phys. Chem. 78 (2009) 976-985. |
| [8] | Y.F. Xu, Y.L. Wang, M.Z. Wang, et al., A new approach of synthesis and morphological control of poly(ethylene terephthalate)-g-polyacrylonitrile composite film with a porous surface, Radiat. Phys. Chem. 106 (2015) 261-267. |
| [9] | J.R. Whinfield, J.T. Dickson, Improvements relating to the manufacture of highly polymeric substances, British Patent, 1941 578079. |
| [10] | G.W. Burton, C.P. O'Farrell, Preparation of artificial latexes, J. Elastomers Plast. 9 (1977) 94-101. |
| [11] | Y. Zhang, H.F. Chan, K.W. Leong, Advanced materials and processing for drug delivery: the past and the future, Adv. Drug Delivery Rev. 65 (2013) 104-120. |
| [12] | L.J. Teng, W.Y. Nie, Y.F. Zhou, L.Y. Song, P.P. Chen, Synthesis and characterization of star-shaped PLLA with sorbitol as core and its microspheres application in controlled drug release, J. Appl. Polym. Sci. 132 (2015) 422131-422137. |
| [13] | J.P. Rao, K.E. Geckeler, Polymer nanoparticles: preparation techniques and sizecontrol parameters, Prog. Polym. Sci. 36 (2011) 887-913. |
| [14] | C.J. Ke, T.Y. Su, H.L. Chen, et al., Smart multifunctional hollow microspheres for the quick release of drugs in intracellular lysosomal compartments, Angew. Chem. Int. Ed. 50 (2011) 8086-8089. |
| [15] | J. Fickert, D. Schaeffel, K. Koynov, K. Landfester, D. Crespy, Silica nanocapsules for redox-responsive delivery, Colloid Polym. Sci. 292 (2014) 251-255. |
| [16] | R.H. Staff, P. Rupper, I. Lieberwirth, K. Landfester, D. Crespy, Phase behavior of binary mixtures of block copolymers and a non-solvent in miniemulsion droplets as single and double nanoconfinement, Soft Matter 7 (2011) 10219-10226. |
| [17] | J. Fickert, C. Wohnhaas, A. Turshatov, K. Landfester, D. Crespy, Copolymers structures tailored for the preparation of nanocapsules, Macromolecules 46 (2013) 573-579. |
| [18] | T. Yamagami, Y. Kitayama, M. Okubo, Preparation of stimuli-responsive "mushroom-like" Janus polymer particles as particulate surfactant by site-selective surface-initiated AGET ATRP in aqueous dispersed systems, Langmuir 30 (2014) 7823-7832. |
| [19] | B. Gupta, N. Muzyyan, S. Saxena, N. Grover, S. Alam, Preparation of ion exchange membranes by radiation grafting of acrylic acid on FEP films, Radiat. Phys. Chem. 77 (2008) 42-48. |
| [20] | D. Campbell, K. Araki, D.T. Turner, ESR study of free radicals formed by γ-irradiation of poly(ethylene terephthalate), J. Polym. Sci., A: Polym. Chem. 4 (1966) 2597-2606. |
| [21] | J. Brandrup, E.H. Immergut, E.A. Grulke, A. Abe, D.R. Bloch, Polymer Handbook, fourth ed., John Wiley & Sons, Inc, New York, NY, 2000. |
 2016, Vol.27
2016, Vol.27 


