b Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, China
Over the past decades,host-guest system with novel topological structures has become a popular research field in supramolecular chemistry [1]. As a type of classic topological structure,the mechanically interlocked molecules such as rotaxanes and catenanes have unique structural features and can be applied in various fields,including molecular devices,molecular switches and machines,nanotechnology,biological technology,drug delivery and polymer materials [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20].
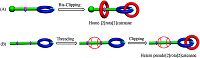
Accordingly,the design and construction of mechanically interlocked molecules with multiple topology units has become one of the most important projects. For example,rotaxane may fuse catenane to compose the corresponding rotacatenane integration [21, 22, 23, 24]. In recent years,rotacatenanes have also attracted increasing interests owing to the fact that it can lead to different chemical structures with interesting functionalities [21, 22, 23, 24]. Motivated by the advancements of our previous works [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35],we recently reported a type of novel rotacatenane topological structures,as shown in Scheme 1(A) [36]. In which,the ammonium template was composed of two different types of ammoniums. One type lay on the linear component of backbone and the other type located on the macrocyclic component of backbone. The guest was employed to perform the one-step template-directed clipping reaction,affording the homo [n]rota[n]catenane with novel topological structure [36]. It prompts us to have an interest to construct the hetero rotacatenane by use of different macrocyclic hosts.
Cucurbituril as a classic macrocyclic host has been widely applied in the construction of pseudorotaxanes [37]. In our previous works,we have confirmed that no dethreading process took place in formation of cucurbituril-based pseudorotaxanes due to the strong binding affinity [38, 39]. Moreover,an efficient synthetic method for the synthesis of hetero[4]rotaxane was reported by Wu using a new "threading-stoppering-followed byclipping" approach [40]. Based on the above consideration,herein, we designed and synthesized a novel ammonium backbone,which contained three ammonium units. Two ammoniums on the linear component of backbone acted as the function with construction of cucurbituril-based pseudorotaxane while another ammonium on the macrocyclic component of backbone played the role of construction of crown ether-based catenane. Therefore,it can be used to construct the hetero pseudo[2]rota[2]catenane with two different macrocyclic hosts by a "threading-followed-by-clipping" approach as shown in Scheme 1(B).
2. ExperimentalAll manipulations were carried out under an argon atmosphere by using standard Schlenk techniques,unless otherwise stated. THF was distilled under nitrogen from sodium to benzophenone. EtOH and MeOH were distilled under drying pipe from magnesium to iodine. DMF was dried with magnesum sulfate and then distilled under a vacuum. NMR spectra were collected with either a 400 or 600 MHz spectrometer. Mass spectra were measured in the ESI or MALDI-TOF mode. Elemental analyses were performed by investigation of C,H and N.
|
Download:
|
| Scheme 1.Schematic representation of homo [2]rota[2]catenane (A) and hetero pseudo[2]rota[2]catenane. | |
Synthesis of compound 3: To a solution of compound 1 (0.50 g, 0.72mmol) in anhydrous MeOH (80 mL) was added compound 2 (0.22 g,1.08mmol)with anhydrous sodiumsulfate acting as drying agent under argon atmosphere. The mixture was refluxed for 24 h. After removing the solvent,and the residue was dissolved in THF (30 mL) andMeOH (30 mL),and then NaBH4 (0.15 g,4.0 mmol)was added slowly in 10 portions. After stirring overnight,the reaction was quenched with the saturated ammonium chloride (aq). The solvents were removed under a vacuum,and the residue was extracted by absolute ethyl ether and then dried over anhydrous sodium sulfate. Solvent was removed under reduced pressure and dried. The unpurified product was dissolved in dry chloroform (60 mL),and then Boc2O (0.62 g,2.88mmol) and triethylamine (0.62 mL)were added. Themixturewas stirred at roomtemperature for24 h. Removal of solventunder reduced pressure and purification on a silica gel column using DCM/ethyl acetate (5:1) as the eluent obtained the Boc-protected amine 3 as a yellowliquid. Yield: 0.49 g, 65%. Compound 3: 1H NMR (600MHz,CDCl3): δ 7.31-7.30 (m,2H), 7.26-7.24 (m,3H),7.02 (s,2H),6.88 (s,2H),6.80 (s,4H),6.39 (s,2H), 6.35 (s,1H),4.39 (s,2H),4.33 (s,2H),4.29 (s,2H),4.24 (s,2H),4.13 (s, 4H),4.05 (s,4H),3.84 (s,8H),3.73 (s,8H),3.18-3.05 (m,4H),1.64 (s, 4H),1.50-1.41 (m,27H),1.20 (s,4H); 13C NMR (100 MHz,CDCl3): δ 159.87,157.71,1=.90,1=.40,130.18,129.94,129.68,128.85, 128.32,127.53,126.95,114.53,106.22,105.60,100.00,79.76,79.40, 70.79,69.71,69.61,67.43,67.25,49.34,46.17,28.36,26.53.; ESI-MS m/z 1108.7 [M+Na+],calculated 1085.6.; Anal. Calcd. for C61H87N3O14: C,67.35,H,8.14,N,3.95. Found: C,67.44,H,8.07, N,3.87.
Synthesis of compound 4: To a solution of the Boc-protected amine 3 (0.22 g,0.2 mmol) in dry DCM (10 mL),TFA (0.37 mL, 6.0 mmol) was added at room temperature. After stirring for 2 h under nitrogen atmosphere,the solvent was removed under a vacuum. The residue was dissolved in MeOH (1.0 mL),and then saturated NH4PF6 (2.0 mL,aq.) was added to yield a brown precipitate. After filtering,washing with H2O and drying under a vacuum,the title compound was obtained as the light yellow gum. Yield: 0.22 g,89%. Compound 4: 1H NMR (600 MHz,CD3CN): δ 7.48 (s,5H),7.29 (d,4H,J = 6.0 Hz),6.97 (d,4H,J = 12.0 Hz),6.= (s,2H), 6.45 (s,1H),4.16 (s,8H),4.09 (s,4H),4.02 (s,4H),3.77-3.72 (m, 8H),3.61 (d,8H,J = 6.0 Hz),3.02-2.97 (m,4H),1.63 (s,4H),1.35 (s, 4H),13C NMR (100 MHz,CD3CN): δ 160.83,160.28,133.31,132.26, 131.14,130.=,130.22,129.62,123.10,115.=,108.67,70.91, 69.64,68.18,51.89,48.17,25.76. ESI-MS m/z 786.5 [M-PF6--2HPF6],calculated 1223.4.,Anal. Calcd. for C46H66F18N3O8P3: C, 45.07,H,5.36,N,3.52. Found: C,45.14,H,5.44,N,3.43.
Synthesis of pseudo[2]rotaxane 5: To a solution of compound 3 (0.22 g,0.2 mmol) in dry DCM (10 mL),trifluoroacetic acid (0.37 mL,6.0 mmol) was added and the mixture was stirred for 4 h at room temperature under nitrogen atmosphere,and then the solvent was removed under a vacuum. Then to a solution of the residue in H2O,cucurbit[6]uril (0.24 g,0.24 mmol) was added and the mixture was refluxed for 24 h under nitrogen atmosphere. After cooling,the mixture was filtered and the filtrate was treated with saturated NH4PF6 (6 mL,aq.) to yield a white precipitate. The solid was collected by filtration,washed with water and dried under vacuum to give the title compound 5 in a yield of 87%. The compound 5: 1H NMR (400 MHz,CD3CN): δ 7.64 (s,2H),7.44 (s, 3H),7.38 (d,4H,J = 8.0 Hz),7.01 (d,4H,J = 8.0 Hz),6.70 (s,2H),6.= (s,1H),5.74 (d,12H,J = 16.0 Hz),5.40 (s,12H),4.26 (m,12H),4.18 (s,4H),4.11 (s,8H),4.02 (s,4H),3.78 (s,8H),3.64 (s,8H),3.20-2.98 (m,4H),0.91-0.76 (m,4H),0.76-0.51 (m,4H). The 13C NMR spectrum was not collected due to the poor solubility of pseudo[2]rotaxane 5; MALDI-MS m/z 1782.7 [M-PF6 --2HPF6],calculated 2220.2.
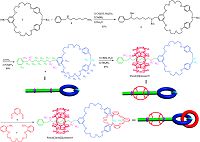
3. Results and discussionThe synthetic route of hetero pseudo[2]rota[2]catenane 8 was outlined in Scheme 2. In the presence of anhydrous sodium sulfate, compound 1 [36] as starting material was treated with 1,6-hexamethylenediamine 2 to afford the corresponding dynamic imine,which was then reduced by NaBH4 in the solution of THF and MeOH to give the kinetically stable amine,and then the NH of free amines was protected by Boc2O. Subsequently,the Bocprotected macrocyclic compound 3 was obtained in 65% yields for three steps. And then the Boc groups were removed with excess trifluoroacetic acid (TFA) in dry dichloromethane. Protonation of the as-formed amine and subsequent counterion exchange with saturated NH4PF6 aqueous solution afforded the target ammonium 4 in 89% yields for two steps. The structure of 4 was well determined by NMR spectroscopy,mass spectrometry and elemental analysis.
|
Download:
|
| Scheme 2.The synthesis of the hetero pseudo[2]rota[2]catenane |
|
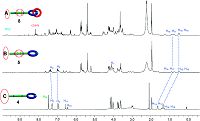
Subsequently,the ammonium 4 was subjected to perform the threading with cucurbituril in H2O,generating the pseudo[2]rotaxane 5 in a yield of 87%. The threading process of cucurbit[6]uril was followed by 1H NMR spectroscopy. As shown in Fig. 1,a series of obvious upfield shifts of proton resonance signals (H14,H15,H16 and H17) on the alkyl chain between two ammoniums were observed,attributing to the shielding effect of the CB [6] host component. The result clearly confirmed the formation of pseudo[2]rotaxane. Furthermore,the result of MALDI-TOF mass spectrometric analysis further supported the assigned structure of pseudo[2]rotaxane 5,and a peak at m/z 1782.7 for [M-PF6 --2HPF6] was observed (Supporting information).
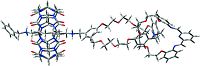
The pseudo[2]rotaxane 5 including dialkylammonium recognition sites on the macrocyclic component was treated with 2,6- pyridinedicarboxaldehyde 6 and the tetraethylene glycol bis(2- aminophenyl)ether 7 in CD3CN. And then a light yellow solution was observed as a result of the formation of dynamic imine,which was well in agreement with experimental phenomena reported in previous literatures [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]. Likewise,the process of clipping reaction was recorded by 1H NMR spectroscopy. As shown in Fig. 1, a characteristic resonance signal of ammonium NH2+ protons on the macrocyclic component as a broad peak at 9.72 ppm was observed along with the occurrence of imine resonance signal at 8.16 ppm. Similar to our previous work [40],the result indicated that a selective clipping reaction took place in the ammonium site on the macrocyclic component of backbone,generating a dynamic hetero pseudo[2]rota[2]catenane 8. Further proof was obtained by the MALDI-TOF mass spectrometry. A peak at m/z 2258.7 for [M-PF6--2HPF6] was observed,which clearly confirmed the existence of hetero pseudo[2]rota[2]catenane 8. For the structure of hetero pseudo[2]rota[2]catenane 8,we employed Gaussian 09 programs to optimize and obtain the energy-minimized structure via the density functional theory (DFT) calculation at the B3LYP/6-31G* level,as shown in Fig. 2.
|
Download:
|
| Fig. 1.1H NMR spectra (600 MHz, CD3CN, 298 K) of |
|
|
Download:
|
| Fig. 2.The energy-minimized structure of hetero pseudo[2]rota[2]catenane |
|
In conclusion,a novel ammonium backbone containing two ammoniums on the linear component and an ammonium on the macrocyclic component was developed. It can be employed to construct the hetero pseudo[2]rota[2]catenane with two different macrocyclic hosts by a "threading-followed-by-clipping" approach. The research provides a foundation for future studies aimed at constructing a complicated integrated structure or supramolecular polymer by use of multiple mechanically interlocked frameworks.
AcknowledgmentsThe authors acknowledge financial support from National Natural Science Foundation of China (No. 21402057),the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education and the self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (Nos. CCNU14A05009,CCNU14F01003).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.010.
| [1] | V. Balzani, A. Credi, M. Venturi, Molecular Devices and Machines:Concepts and Perspectives for the Nanoworld, 2nd ed., Wiley-VCH, Weinheim, 2008. |
| [2] | A.R. Pease, J.O. Jeppesen, J.F. Stoddart, et al., Switching devices based on interlocked molecules, Acc. Chem. Res. 34(2001) 433-444. |
| [3] | S. Saha, J.F. Stoddart, Photo-driven molecular devices, Chem. Soc. Rev. 36(2007) 77-92. |
| [4] | A.C. Fahrenbach, S.C. Warren, J.T. Incorvati, et al., Organic switches for surfaces and devices, Adv. Mater. 25(2013) 331-348. |
| [5] | J.A. Faiz, V. Heitz, J.-P. Sauvage, Design and synthesis of porphyrin-containing catenanes and rotaxanes, Chem. Soc. Rev. 38(2009) 422-442. |
| [6] | J.E. Beves, B.A. Blight, C.J. Campbell, Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links, Angew. Chem. Int. Ed. 50(2011) 9260-9327. |
| [7] | A. Coskun, M. Banaszak, R.D. Astumian, et al., Great expectations:can artificial molecular machines deliver on their promise, Chem. Soc. Rev. 41(2012) 19-30. |
| [8] | V.N. Vukotic, S.J. Loeb, Coordination polymers containing rotaxane linkers, Chem. Soc. Rev. 41(2012) 5896-5906. |
| [9] | D.H. Qu, H. Tian, Novel and efficient templates for assembly of rotaxanes and catenanes, Chem. Sci. 2(2011) 1011-1015. |
| [10] | S. Saha, K.C.F. Leung, T.D. Nguyen, et al., Nanovalves, Adv. Funct. Mater. 17(2007) 685-693. |
| [11] | V. Sindelar, S. Silvi, S.E. Parker, et al., Proton and electron transfer control of the position of cucurbit[n]uril wheels in pseudorotaxanes, Adv. Funct. Mater. 17(2007) 694-701. |
| [12] | I. Willner, B. Basnar, B. Willner, From molecular machines to microscale motility of objects:application as "smart materials", sensors, and nanodevices, Adv. Funct. Mater. 17(2007) 702-717. |
| [13] | Y. Chen, Y. Liu, Cyclodextrin-based bioactive supramolecular assemblies, Chem. Soc. Rev. 39(2010) 495-505. |
| [14] | J. Zhang, B. Han, Y. Zhao, et al., CO2 capture by hydrocarbon surfactant liquids, Chem. Commun. 47(2011) 1033-1035. |
| [15] | X. Yan, F. Wang, B. Zheng, et al., Stimuli-responsive supramolecular polymeric materials, Chem. Soc. Rev. 41(2012) 6042-6065. |
| [16] | J.M. Yi, X.L. Ni, X. Xiao, et al., Complexation of sym-bis(benzimidazole)-2,20-ethylene salts with cucurbit[6] uril derivatives:a potential axle molecule for pseudorotaxanes, Chin. Chem. Lett. 24(2013) 362-366. |
| [17] | Y. Han, Y. Jiang, C.F. Chen, Solid state self-assembly of triptycene-based catechol derivatives by multiple O-H…O hydrogen bonds, Chin. Chem. Lett. 24(2013) 475-478. |
| [18] | H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24(2013) 545-552. |
| [19] | H. Wang, Z.J. Zhang, H.Y. Zhang, et al., Synthesis of a bistable[3] rotaxane and its pH-controlled intramolecular charge-transfer behavior, Chin. Chem. Lett. 24(2013) 563-567. |
| [20] | S. Sun, J.B. Shi, Y.P. Dong, et al., A pillar[5] arene-based side-chain pseudorotaxanes and polypseudorotaxanes as novel fluorescent sensors for the selective detection of halogen ions, Chin. Chem. Lett. 24(2013) 987-992. |
| [21] | D.B. Amabilino, P.R. Ashton, J.A. Bravo, et al., Template-directed synthesis of a rotacatenane, Eur. J. Org. Chem.(1999) 1295-1302. |
| [22] | G. Barin, A. Coskun, D.C. Friedman, et al., A multistate switchable[3] rotacatenane, Chem. Eur. J. 17(2011) 213-222. |
| [23] | R.S. Forgan, J.J. Gassensmith, D.B. Cordes, et al., Self-assembly of a[2] pseudorota[3] catenane in water, J. Am. Chem. Soc. 134(2012) 17007-17010. |
| [24] | R. Hayashi, K. Wakatsuki, R. Yamasaki, et al., Synthesis of rotacatenanes by the combination of Cu-mediated threading reaction and the template method:the dual role of one ligand, Chem. Commun. 50(2014) 204-206. |
| [25] | J. Yin, S. Dasgupta, J. Wu, Synthesis of[n]rotaxanes by template-directed clipping:the role of the dialkylammonium recognition sites, Org. Lett. 12(2010) 1712-1715. |
| [26] | Z. Li, W. Liu, J. Wu, et al., Synthesis of[2] catenanes by template-directed clipping approach, J. Org. Chem. 77(2012) 7129-7135. |
| [27] | Z. Li, G. Liu, W. Xue, et al., Construction of hetero[n]rotaxanes by use of polyfunctional rotaxane frameworks, J. Org. Chem. 78(2013) 11560-11570. |
| [28] | G. Liu, Z. Li, D. Wu, et al., Dendritic[2] rotaxanes:synthesis, characterization, and properties, J. Org. Chem. 79(2014) 643-652. |
| [29] | Z. Li, F. Hu, G. Liu, et al., Photo-responsive[2] catenanes:synthesis and properties, Org. Biomol. Chem. 12(2014) 7702-7711. |
| [30] | F. Hu, J. Huang, J. Cao, et al., Dithienylethene-based rotaxanes:synthesis, characterization and properties, Org. Biomol. Chem. 12(2014) 7712-7720. |
| [31] | Z.Y. Li, W. Xue, G.X. Liu, et al., Synthesis and properties of template-promoted switchable dithienylethene-based macrocycles, Chin. Chem. Lett. 24(2013) 189-191. |
| [32] | G. Liu, D. Wu, J. Liang, et al., Tetraphenylethene modified[n]rotaxanes:synthesis, characterization and aggregation induced emission behavior, Org. Biomol. Chem. 13(2015) 4090-4100. |
| [33] | Z. Li, X. Han, H. Chen, et al., Construction of photoswitchable rotaxanes and catenanes containing dithienylethene fragments, Org. Biomol. Chem. 13(2015) 7313-7322. |
| [34] | X. Han, F. Hu, H. Ge, et al., The application of template-directed clipping approach in constructing mechanically interlocked molecules based on N-hetero crown ethers, Prog. Chem. 27(2015) 675-686. |
| [35] | X. Han, M. Cao, Z. Xu, et al., Aggregation-induced emission behavior of pHcontrolled molecular shuttle based on tetraphenylethene moiety, Org. Biomol. Chem. 13(2015) 9767-9774. |
| [36] | W. Xue, Z. Li, G. Liu, et al., Construction of rotacatenanes using rotaxane and catenane frameworks, Org. Biomol. Chem. 12(2014) 4862-4871. |
| [37] | K. Kim, Mechanically interlocked molecules incorporating cucurbituril and their supramolecular assemblies, Chem. Soc. Rev. 31(2002) 96-107. |
| [38] | J. Yin, C. Chi, J. Wu, Efficient preparation of separable pseudo[n]rotaxanes by selective threading of oligoalkylammonium salts with cucurbit[7] uril, Chem. Eur. J. 15(2009) 6050-6057. |
| [39] | M. Cao, F. Hu, X. Han, et al., Aggregation control of hemicyanine fluorescent dye by using of cucurbit[7] uril and pillar[6] arene, Chin. J. Chem. 33(2015) 351-355. |
| [40] | J. Yin, C. Chi, J. Wu, Efficient synthesis of a hetero[4] rotaxane by a "threadingstoppering-followed-by-clipping" approach, Org. Biomol. Chem. 8(2010) 2594-2599. |
 2016, Vol.27
2016, Vol.27 


