b College of Environmental and Chemical Engineering, Heilongjiang University of Science and Technology, Harbin 150022, China
Dye-sensitized solar cells (DSSCs) have attracted much attention due to their low-cost production,non-vacuum process, environment friendly energy conversion,and fairly high performance since the outstanding breakthroughs made by the Grätzel group [1, 2, 3, 4, 5, 6, 7, 8]. However,much improvement in efficiency is desired for these devices before they could take place of the Si based solar cells. Therefore,research groups all over the world have provided several strategies to improve the photovoltaic performance of ruthenium dye sensitized solar cells,among which co-sensitization is considered to be a more promising way to combine the spectral responses of co-adsorbents on the film for performance enhancement [9, 10, 11]. For example,Han and Arakawa obtained a high efficiency of above 11.0% by co-sensitization of black dye and organic compounds,respectively [12, 13]. Dehghani co-sensitized N719 dye with a porphyrin dye ZnTCPP,resulting in the efficiency of 6.35%,which was higher than that of 4.75% based on N719 alone [14]. Holliman co-sensitized N719 dye with triarylamine dyes, achieving efficiency of 7.5% which exceeds those records for individual dye devices [15]. Yang reported a co-sensitized system using N719 dye with metal-free organic dye FL. The cell efficiency was optimized to be 5.10%,which was higher than that of the cell soaking only in N719 (4.89%) [16]. Ko published a co-sensitization study using Ru-complex JK-142 and organic JK-62 dye. The DSSC efficiency exhibited 10.2% surpassing that of cells using only JK- 142 dye (7.28%) or JK-62 dye (5.36%) [17]. Sharma obtained a cell efficiency of 7.35% by co-sensitization of zinc-porphyrin and thiocyanate-free ruthenium (II) terpyridine dyes [18]. Wu cosensitized N719 with an organic dye resulting in an efficiency of 7.91%,8.6% higher than that of N719 sensitized alone [19]. Chang reported efficient panchromatic light harvesting by co-sensitization of a porphyrin molecule and N719 in dye-sensitized solar cells, the co-sensitized devices shows a considerably enhanced power conversion efficiency of 8.89%,higher than those individually sensitized by N719 [20]. However,the selection of co-adsorbent is very important in this co-sensitization. It should have a large molar extinction coefficient in the infrared region or around 400 nm to recover the dip in the IPCE spectra induced by I3-,a suitable molecular structure to avoid competitive adsorption among dyes while effectively suppressing the aggregation of dyes on the TiO2 surface,and it should be able to reduce the recombination of electrons in the TiO2 film with I3- by the formation of a compacted molecule monolayer covering the bare TiO2 surface.
Therefore,in this work,we synthesized a new co-adsorbent of N,N'-bis((pyridin-2-yl)(methyl)methylene)-o-phenylenediamine (named BPPI,Scheme 1) and employed it into ruthenium dye N719 (Scheme 1) sensitized DSSCs. BPPI is a co-adsorbent with a suitable molecular structure which will not competitively adsorb with N719,it contains pyridine-anchor unit instead of the conventional carboxyl-anchor group and this pyridine-anchor co-adsorbent is confirmed to adsorb preferentially at the Lewis acid sites of the TiO2 surface (the sites of exposed Ti atoms) [21, 22]. What is more, it has a large molar extinction coefficient around 400 nm which could recover the dip in the IPCE spectra induced by I3-. By cosensitizing this co-adsorbent with N719,the co-sensitized device exhibited enhancements of photovoltaic performance. Mechanistic investigations were carried out by various spectral and electrochemical characterizations.
|
Download:
|
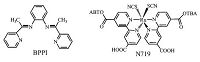
| Scheme 1.Molecular structures of BPPI and N719. | |
The FTO conducting glass (Fluorine-doped SnO2,sheet resistance 15 Ω per square,transmission 90% in the visible range) was purchased from NSG,Japan,and cleaned by a standard procedure. N719 [Bis-tetrabutylammonium cis-bis(isothiocyanato) bis(2,2- bipyridyl-4,4-dicarbox-ylato)ruthenium(II)] was purchased from Solaronix Company,Switzerland. All the other solvents and chemicals used in this work were of reagent grade without further purification. And all characterizations were carried out under ambient pressure and room temperature.
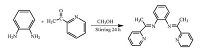
2.2. Synthesis of BPPIThe synthesis process of BPPI is shown in Scheme 2. Typically, 1,2-diaminobenzene (1081 mg,10 mmol) was added into anhydrous methanol (10 mL),and the mixed solution was allowed to stir magnetically for 10 min. 2-Acetylpyiridine (2.243 mL,20 mmol) was added to the mixture under an inert atmosphere and allowed to further stir for 24 h at room temperature. There was precipitate from the pale yellow solution. After filtering,BPPI was isolated. Yield: 142.3 mg(45%); UV/vis (CH2Cl2):λmax 240,316,and 345 nm; FT-IR (KBr,cm-1): ν 3052 (s,νPy,C-H),2977 (w),1595 (s,νC=N),1569 (s),1439 (s),1400 (s),1316 (s),1277 (s),1153 (m),991 (m),744 (vs), 698 (m),614 (m),536 (m); 1H NMR (400 MHz,DMSO-d6,298 K, TMS): δ 8.98 (m,1H,Py-H),8.= (m,1H,Ph-H),8.12 (m,1H,Ph-H), 7.96 (m,1H,Py-H),7.84 (m,1H,Py-H),7.= (m,1H,Py-H),7.51-6.16 (m,4H,Py-H),1.68-1.53 ppm (m,6H,CH3); elemental analysis calcd (%) for C20H18N4: C 76.41,H 5.77,N 17.82; found: C 76.68,H 5.91,N 17.41.
|
Download:
|
| Scheme 2.Synthesis route of N,N'-bis((pyridin-2-yl)(methyl)methylene)-o-phenylenediamine. | |
Dye-sensitized solar cells were fabricated using the following procedure. The TiO2 paste was cast onto the FTO substrate by the screen-printing method,followed by drying at 100 ℃ for 5 min, and this process was repeated for six times,followed by sinterings at 500 ℃ for 15 min in air to obtain a transparent TiO2 photoelectrode with the thickness of ca. 10 μm. The co-sensitized electrodes were prepared by immersing the obtained mesoporous TiO2 photoelectrode into 0.3 mmol L-1 BPPI solution in absolute ethanol for 2 h. The electrode was then washed with ethanol and dried. A second immersion in 0.3 mmol L-1 N719 solution in absolute ethanol for 12 h was followed by washing with ethanol and drying again. For the co-adsorbed solar cells,chenodeoxycholic acid (CDCA) was added into the dye solutions at a concentration of 10 mmol L-1. The single N719 sensitized electrodes were prepared by only immersing TiO2 photoelectrode into 0.3 mmol L-1 N719 solution in absolute ethanol for 14 h. The electrolyte used in this work was 0.5 mol L-1 LiI + 0.05 mol L-1 I2 + 0.1 mol L-1 tert-butylpyridine in a 1:1 (volume ratio) of acetonitrile-propylene carbonate. The platinum counter electrode was prepared by depositing H2PtCl6 paste onto the FTO glass substrates and then sintering at 450 ℃ for 30 min. The cells were assembled by sandwiching the electrolyte between the dye sensitized photoanode and the counter electrode,and the assembly was held together using mini-binder clips.
2.4. MeasurementsUV-visible absorption spectra in ethanol solution were recorded on a SPECORD S600 UV-visible spectrophotometer (Jena, Germany),and absorption spectra of TiO2 films were recorded on a UV-2250 spectrophotometer (Shimadzu,Japan). 1H NMR (400 MHz) spectra were recorded on a Bruker Avance-400 spectrometer using Si(CH3)4 as an internal standard at room temperature. Infrared spectra (IR) were obtained from KBr pellets on a Nicolet Avatar-360 Ingrared spectrometer in the 4000- 100 cm-1 region. Elemental analyses were performed on a Perkin- Elmer 2400 element analyzer. The cyclic voltammetry (CV) were measured with a electrochemical workstation (CHI660d,Chenhua, Shanghai) using a three-electrode cell with a Pt working electrode, a Pt wire auxiliary electrode,and a saturated calomel reference electrode in saturated KCl solution. The supporting electrolyte was 0.1 mol L-1 tetrabutylammonium hexafluorophosphate (TBAPF6, Fluka,electrochemical grade) in ethanol as the solvent. Photocurrent- photovoltage (I-V) curves were recorded by Keithley model 2400 digital source meter using a mask with an aperture area of 0.16 cm2. The irradiance of AM1.5 global sunlight from a filtered 500W xenon lamp light source was set at 100mW cm-2 and was calibrated by a standard silicon solar cell (NO. NIMMS1123, calibrated by National Institute of Metrology,China). Based on I-V curve,the fill factor (FF) is defined as: FF = (Jmax × Vmax)/(Jsc × Voc), where Jmax and Vmax are the photocurrent density and photovoltage for maximum power output; Jsc and Voc are the shortcircuit photocurrent density and open-circuit photovoltage, respectively. The overall energy conversion efficiency η is defined as: η = (FF × Jsc × Voc)/Pin where Pin is the power of the incident light. IPCE was measured on an EQE/IPCE spectral response system (Newport). The SPS instrument was assembled by Jilin University, in which monochromatic light was obtained by passing light from a 500W xenon lamp through a double-prism monochromator (SBP300,China),and the signal were collected by a lock-in amplifier (SR830,Stanford). EIS were recorded by CHI660D Electrochemical Analyzer (Chenhua,China),and the measurements were taken over a frequency range of 0.1-100 kHz under standard global AM 1.5 solar irradiation by applying a forward bias of -0.75 V. Open-circuit voltage decay curves (OCVD) and dark current were also recorded by CHI660D Electrochemical Analyzer.

|
Download:
|
| Fig. 1.UV-visible absorption spectra of BPPI and N719 (a) in ethanol, (b) on TiO2 film. | |
The absorption spectrum of BPPI in ethanol and on film is shown in Fig. 1,and its absorption data are listed in Table 1. In ethanol solution,BPPI displays a strong absorption peak at ca. 2= nm with a weak shoulder absorption band at 377 nm. Compared with the absorption spectrum of N719,the absorption spectra of BPPI could compensate for that of N719 in the low wavelength region of visible spectrum,especially in the region of 300-450 nm. Furthermore,as shown in Table 1,the molar extinction coefficients of BPPI is much higher than that of the ruthenium complex N719 and I3- (25,000 dm3 mol-1 cm-1, 380 nm) [23, 24]. A higher molar extinction coefficient means a higher light harvesting ability in the wavelength region of 300- 450 nmcompared with N719 and I3-. Hence,the photon lost due to the light absorption by I3- will be suppressed by employing BPPI as a co-adsorbent due to the competition between BPPI and I3- to absorb light. When absorbed on TiO2 film,the absorption of N719 is remarkably broadened due to the electronic coupling of the dyes on the TiO2 surface [25]. After co-adsorption with BPPI,its absorption was enhanced in the region of 300-700 nm,especially around 400 nm where BPPI could compensate for that of N719. From the adsorption spectra,it could be predicted that employing BPPI into DSSCs as a co-adsorbent is an effective way to enhance its performance by overcoming the deficiency of N719 absorption in the low wavelength region,offsetting competitive visible light absorption of I3-,and enhancing the spectral responses of the co-sensitized TiO2 film.
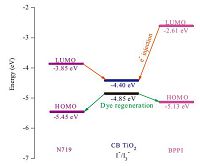
3.2. Electrochemical propertiesEnergy matching is crucial in selecting sensitizers for DSSCs. To estimate the energy level of BPPI,cyclic voltammetry (CV) experiments were carried out and the data from CV experiments are summarized in Table 1. Based on their first oxidation potentials,the HOMO value for BPPI is -5.13 eV [26]. As calculated from the intersection between absorption and emission spectra, the excitation transition energy (E0-0) for BPPI is 2.52 eV. Therefore, the LUMO levels of BPPI,calculated from EHOMO + E0-0,is -2.61 eV. For better electron injection,this LUMO level should lie above the conduction band (CB) of the TiO2 semiconductor (-4.0 eV vs. vacuum) and for effective dye regeneration,the HOMO energy level should lie below the I-/I3- redox electrolyte (-4.6 eV vs. vacuum) which is further improved negatively about 0.3 V by adding additives such as 4-tert-butylpyridine (TBP) to the I-/I3- redox electrolyte [27]. Hence,as shown in Fig. 2,the energy levels of BPPI are suitable for electron injection and dye regeneration thermodynamically [28].
|
|
Table 1 Experimental data for spectral and electrochemical properties of BPPI. |
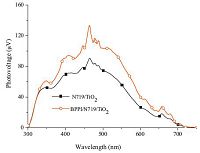
The surface photovoltage spectrum (SPS) method is commonly applied in DSSCs based on TiO2,which is a well-established contactless technique for surface state distribution [29, 30]. The SPS of photoanodes individually sensitized by N719 sensitizer and co-sensitized by N719 and BPPI are shown in Fig. 3. As shown in Fig. 3,co-adsorption could enhance the photovoltage signals of the sensitized films between 300 and 750 nm,which indicates that the separate efficiency of electrons and holes correlate strongly with BPPI. Combining the results of UV-visible absorption spectra and SPS,it is easy to find that the spectral response of the N719 sensitized TiO2 photoelectrodes is enhanced by co-adsorbing with BPPI.
|
Download:
|
| Fig. 2.Energy levels of BPPI and of the other materials used for DSSCs fabrication. | |
|
Download:
|
| Fig. 3.SPS of co-sensitized photoelectrodes and N719 sensitized photoelectrode. | |
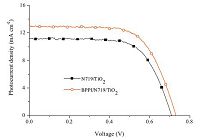
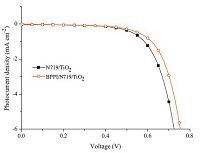
To investigate the effect of BPPI on the photovoltaic properties of DSSCs,BPPI/N719 co-sensitized DSSCs (denoted as BPPI/N719/ TiO2) were fabricated following a stepwise co-sensitization procedure by sequentially immersing the TiO2 electrode (with thickness of ca. 10 mm) in separate solutions of BPPI and N719. For comparison purpose,a device sensitized by the individual dye of N719 (denoted as N719/TiO2) was also fabricated under the same experimental conditions. The current-voltage (J-V) characteristics of the DSSCs based on different photoanodes under illumination (AM 1.5 G,100mW cm-2) are shown in Fig. 4,and the corresponding cell performances are summarized in Table 2. The individually N719 sensitized device was found to exhibit h value of 5.29% (with Jsc = 11.22 mA cm-2,Voc = 0.71 V,and FF = 0.66); the BPPI/N719 co-sensitized solar cell device showed a h value of 6.22% (with Jsc = 12.98 mA cm-2,Voc = 0.73 V,and FF = 0.66).
|
Download:
|
| Fig. 4.J-V curves for DSSCs based on different photoelectrode. | |
|
|
Table 2 Performances of DSSCs based on different photoelectrodes. |
The higher η value of co-sensitized solar cell compared with the individually N719 sensitized devices is attributed to the enhanced photovoltaic parameters of Jsc and Voc. Obviously,the enhanced Jsc value is ascribed to the enhanced IPCE response of the cell,since they are related by the equation:

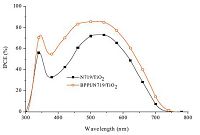
where,e is the elementary charge and φphAM1.5G is the photon flux at AM 1.5 G [31],the enhanced Jsc value is ascribed to the enhanced IPCE response of the co-sensitized cell. Fig. 5 collected the IPCE spectra of the two devices. It is found that co-adsorption with BPPI could enhance the spectral response of N719 on TiO2 film in the whole visible region and consequently enhance the photocurrent performance. This means the co-sensitization of N719 and BPPI has a significant synergy effect on light harvesting, electron injection,and electron collection on TiO2. Based on the IPCE and the absorption spectra,it could be concluded that the cell's higher Jsc in the case of co-sensitization is ascribed to the compensated adsorption of N719 on the TiO2 film,the enhanced spectral responses of co-sensitized TiO2 film,and the offset competitive absorption of I3- around 400 nm.
|
Download:
|
| Fig. 5.IPCE spectra of DSSCs based on single N719 sensitized and co-sensitized photoelectrodes. | |
On the other hand,dye aggregation is an important factor that affects the performance of DSSCs. In a co-sensitization system,all the dyes should have a suitable molecular structure to avoid competitive adsorption and to effectively suppress the aggregation of dyes on the TiO2 surface. Therefore,in order to determine whether the aggregation of BPPI and N719 occurs on the surface of TiO2 electrode,a widely used anti-aggregation co-adsorbent, chenodeoxycholic acid (CDCA) [32, 33, 34],was also applied in N719 sensitized DSSCs with BPPI,and the results are also listed in Table 2. When CDCA was present in the co-sensitized devices of BPPI/N719,device efficiency was not further improved (η = 6.24, Jsc = 12.89 mA cm-2,Voc = 0.74 V,and FF = 0.65). This indicates that no aggregation occurs in the co-sensitization system. In other words,the molecular structure of BPPI is suitable in this cosensitization system to avoid competitive adsorption and suppress the aggregation of N719.
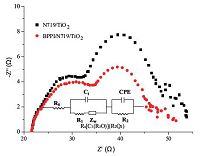
3.5. Electrochemical impedance spectroscopy (EIS)EIS was employed to analyze the charge carrier dynamics in the interfacial regions of solid-liquid layers in DSSCs. The Nyquist plots of EIS for DSSCs sensitized only with N719 and co-sensitized with N719 and BPPI as measured under standard AM1.5G solar irradiation are shown in Fig. 6. Three semicircles in the Nyquist plots are observed. The small and large semicircles located in the high and middle frequency regions are assigned to the charge transfer at the Pt/electrolyte and TiO2/dye/electrolyte interfaces, respectively. Another small semicircle can be assigned to the Nernst diffusion in the electrolyte,which should have appeared at the low frequency region,which is not clear due to overlap with the middle frequency large semicircle. Under light illumination,the radius of the large semicircle located in middle frequency regions in the Nyquist plot decreases after co-adsorption with BPPI, suggesting a decrease of the electron transfer impedance and an increase of charge transfer rate at this interface.
|
Download:
|
| Fig. 6.Nyquist plots of DSSCs based on different photoelectrodes measured under standard AM 1.5G solar irradiation. Insert is the Equivalent circuit used to represent interface in DSSCs. | |
In order to further understand the complex charge transfer process in DSSCs,a physical model has been proposed [35],and the equivalent circuit represented in the insert of Fig. 6, Rs[C1(R1O)](R2Q2),is used to model this system,representing interfaces in composite solar cells. The symbols R and C describe a resistance and a capacitance,respectively,Rs is series resistance,R1 and R2 are the charge transfer resistance,O,which depends on the parameters Y0,1 and B,accounts for a finite-length Warburg diffusion (Zw),and Q is the symbol for constant phase element (CPE,its parameters are Y0,2 and n). The parameters obtained by fitting the impedance spectra of co-sensitized solar cells measured under standard AM1.5 G solar irradiation using the equivalent circuit are listed in Table 3. The series resistance Rs decreases by about 0.4 Ω; the resistance R1 which is estimated from the middle frequency range semicircle in Nyquist plots decreases a lot with the introduction of BPPI,and the resistance R2 which is estimated from the high frequency range semicircle of Nyquist plots also decreases with the introduction of BPPI,and the total resistance of co-sensitized DSSCs decreases compared with single N719 sensitized DSSCs. The total resistance decrease is beneficial for electron transfer and further improving the photocurrent of DSSCs. This suggests that the high Jsc value in DSSC sensitized with BPPI/ N719 is also due to the decrease of internal cell resistance and better charge transfer.
|
|
Table 3 Parameters obtained by fitting the impedance spectra of solar cells measured under standard AM 1.5 G solar irradiation using the equivalent circuit. |
In an effort to understand the enhancement of the Voc value in the co-sensitized solar cell and to investigate the kinetics of recombination processes,electrochemical impedance spectra (EIS) of devices based on different photoelectrodes were measured in the dark by applying a forward bias of -0.75 V [36]. In dark conditions,as shown in Fig. 7a,the three semicircles located in high,middle,and low frequency regions (left to right) of Nyquist plots are attributed to the redox reaction at the Pt counter electrode,the electron transfer at the TiO2/dye/electrolyte interface,and charge transfer in the electrolyte (Nernst diffusion) [37]. Therefore,the larger semicircle observed in the middle frequency region represents the resistances of the charge transfer from the TiO2 to the electrolyte (back recombination resistance Rrec). The radius of this semicircle increases after co-adsorption with BPPI, indicating an increase of Rrec. A large Rrec means a small charge recombination rate and vice versa. The increased value of Rrec for DSSCs implies the retardation of the charge recombination between injected electron and I3- ions in the electrolyte,with a consequent increase of Voc.
The electron lifetime (τe) in different devices were calculated from the Bode phase plots of the EIS spectra of different solar cells (Fig. 7b),according to the relationship:


|
Download:
|
| Fig. 7.Nyquist plots of DSSCs based on different photoelectrodes measured under in dark. | |
Dark current measurement of DSSCs is considered a qualitative technique to describe the extent of the back electron transfer [38, 39]. It could provide useful information regarding the back electron transfer process by making a comparison of dark current between the investigated cells. Therefore,dark current of cells based on N719 and BPPI/N719 were measured and are shown in Fig. 8. By comparing the curves in Fig. 8,it is found that the onset of the dark current for individual N719 sensitized DSSC is at a bias about +0.35 V,and then the dark current increases remarkably with the increase of potential. In contrast,for the BPPI/N719 co-sensitized DSSCs,the onset potential shifted to about + 0.40 V; furthermore,the dark current of the co-sensitized DSSCs increased much slower than that of N719 sensitized DSSC when potentialwas greater than +0.40 V. In otherwords,under the same potential bias,when the potential was ≥0.40 V,the dark current for the co-sensitized DSSCs was noticeably smaller than that for the N719 sensitized DSSC. The increase of the onset potential and the reduction of the dark current demonstrated that BPPI successfully suppresses the electron back reaction with I3- in the electrolyte by forming a compact layer with N719. This is critical to reduce the current leakage in DSSCs and enhance their performance.
|
Download:
|
| Fig. 8.Dark current of the DSSCs based on different photoelectrodes. | |
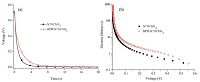
The OCVD technique is a powerful tool to study interfacial recombination processes in the TiO2 DSSCs between injected electrons and the electrolyte. It can provide some quantitative information on the electron recombination velocity in DSSCs [40, 41]. The OCVD decay curves of the DSSCs based on different photoelectrodes are shown in Fig. 9a. It was observed that the OCVD response of the DSSC with BPPI/N719 co-adsorbed photoelectrode was much slower than that individually sensitized byN719,especially in the shorter time domain (within 12 s). Since the decay of the Voc reflects the decrease in the electron concentration,which is mainly caused by the charge recombination [42],the cell sensitized by BPPI/N719 has a lower electron recombination rate than that of the cell individually sensitized by N719.
Under the dark and open-circuit state conditions,electron lifetime (τe) was used to quantify the extent of electron recombination with the redox electrolyte. τe was calculated with the OCVD results in Fig. 9a according to the following equation:

|
Download:
|
| Fig. 9.(a) Open-circuit voltage decay curves of the DSSCs based on different photoelectrodes. (b) Comparison of electron lifetime as a function of open-circuit voltage of DSSCs based on different photoelectrodes. | |
In conclusion,we have developed and investigated N,N'-bis ((pyridin-2-yl) (methyl)methylene)-o-phenylenediamine (named BPPI) as a co-adsorbent in ruthenium dye N719 based solar cell. The co-adsorption could overcome the deficiency of N719 absorption in the low wavelength region of visible spectrum, offset competitive visible light absorption of I3-,enhance the spectral responses of the co-sensitized TiO2 photoelectrode, suppress charge recombination,prolong electron lifetime,and decrease the total resistance of DSSCs. These all help to improve the performance of DSSCs. The device co-adsorbed by BPPI/N719 yields the overall efficiency of 6.22%,which is much higher than that of the device only sensitized by N719 (5.29%). Moreover,the devices in this work were fabricated without scatterings layers, TiCl4 treatment,or anti-reflecting coatings,but could improve the performance of the devices by only simple co-adsorption. This way of simple co-adsorption is a potential method that deserves to be further developed for high efficiency DSSC fabrication.
AcknowledgmentThis work was supported by National Natural Science Foundation of China (Nos. 21171044 and 21371040),the National Key Basic Research Program of China (973 Program,No. 2013CB632900),and the Fundamental Research Funds for the Central Universities (No. HIT. IBRSEM. A201409),also Program for Innovation Research of Science in Harbin Institute of Technology (PIRS of HIT Nos. A201418,A201416 and B201414).
| [1] | A. Hagfeldt, G. Boschloo, L.C. Sun, L. Koo, H. Pettersso, Dye-sensitized solar cells, Chem. Rev. 110(2010) 6595-6663. |
| [2] | B. O'Regan, M. Grätzel, A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 films, Nature 353(1991) 737-740. |
| [3] | P.J. Cameron, L.M. Peter, Characterization of titanium dioxide blocking layers in dye-sensitized nanocrystalline solar cells, J. Phys. Chem. B 107(2003) 14394-14400. |
| [4] | M.K. Nazeeruddin, F. de Angelis, S. Fantacci, et al., Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers, J. Am. Chem. Soc. 127(2005) 16835-16847. |
| [5] | K.M. Guo, M.Y. Li, X.L. Fang, et al., Performance enhancement in dye-sensitized solar cells by utilization of a bifunctional layer consisting of core-shell b-NaYF4:Er3+/Yb3+@SiO2 submicron hexagonal prisms, J. Power Sources 249(2014) 72-78. |
| [6] | Y.R. Gao, L.L. Chu, W. Guo, T.L. Ma, Snythesis and photoelectric properties of an organic dye containing benzo[1, 2-b:4,5-b'] dithiophene for dye-sensitized solar cells, Chin. Chem. Lett. 24(2013) 149-152. |
| [7] | Q.P. Liu, Analysis on dye-sensitized solar cells based on Fe-doped TiO2 by intensity-modulated photocurrent spectroscopy and Mott-Schottky, Chin. Chem. Lett. 25(2014) 953-956. |
| [8] | Y.Q. Wang, X.L. Gao, B. Song, Y.L. Gu, Y.M. Sun, Photoelectrochemical properties of MWCNT-TiO2 hybrid materials as a counter electrode for dye-sensitized solar cells, Chin. Chem. Lett. 25(2014) 491-495. |
| [9] | A. Yella, H.W. Lee, H.N. Tsao, et al., Porphyrin-sensitized solar cells with cobalt(Ⅱ/ⅡI)-based redox electrolyte exceed 12 percent efficiency, Science 334(2011) 629-634. |
| [10] | S.P. Singh, M. Chandrasekharam, K.S.V. Gupta, et al., Co-sensitization of amphiphilic ruthenium(Ⅱ) sensitizer with a metal free organic dye:improved photovoltaic performance of dye sensitized solar cells, Org. Electron. 14(2013) 1237-1241. |
| [11] | G.D. Sharma, M.S. Roy, S.P. Singh, Improvement in the power conversion efficiency of thiocyanate-free Ru(Ⅱ) based dye sensitized solar cells by cosensitization with a metal-free dye, J. Mater. Chem. 22(2012) 18788-18792. |
| [12] | L.Y. Han, A. Islam, H. Chen, et al., High-efficiency dye-sensitized solar cell with a novel co-adsorbent, Energy Environ. Sci. 5(2012) 6057-6060. |
| [13] | H. Ozawa, R. Shimizu, H. Arakawa, Significant improvement in the conversion efficiency of black-dye-based dye-sensitized solar cells by cosensitization with organic dye, RSC Adv. 2(2012) 3198-3200. |
| [14] | M. Mojiri-Foroushani, H. Dehghani, N. Salehi-Vanani, Enhancement of dye-sensitized solar cells performances by improving electron density in conduction band of nanostructure TiO2 electrode with using a metalloporphyrin as additional dye, Electrochim. Acta 92(2013) 315-322. |
| [15] | P.J. Holliman, M. Mohsen, A. Connell, et al., Ultra-fast co-sensitization and trisensitization of dye-sensitized solar cells with N719, SQ1 and triarylamine dyes, J. Mater. Chem. 22(2012) 13318-13327. |
| [16] | K.M. Lee, Y.C. Hsu, M. Ikegami, et al., Co-sensitization promoted light harvesting for plastic dye-sensitized solar cells, J. Power Sources 196(2011) 2416-2421. |
| [17] | S.Q. Fan, C. Kim, B. Fang, et al., Dye heterogeneously positioning on a single TiO2 electrode, J. Phys. Chem. C 115(2011) 7747-7754. |
| [18] | G.D. Sharma, D. Daphnomili, K.S.V. Gupta, et al., Enhancement of power conversion efficiency of dye-sensitized solar cells by co-sensitization of zinc-porphyrin and thiocyanate-free ruthenium(Ⅱ) terpyridine dyes and graphene modified TiO2 photoanode, RSC Adv. 3(2013) 22412-22420. |
| [19] | Z.S. Wu, Y.N. Wei, Z.W. An, X.B. Chen, P. Chen, Co-sensitization of N719 with an organic dye for dye-sensitized solar cells application, Bull. Korean Chem. Soc. 35(2014) 1449-1454. |
| [20] | S. Chang, H.D. Wang, L.T.L. Lee, et al., Panchromatic light harvesting by N719 with a porphyrin molecule for high-performance dye-sensitized soalr cells, J. Mater. Chem. C 2(2014) 3521-3526. |
| [21] | Y. Harima, T. Fujita, Y. Kano, et al., Lewis-acid sites of TiO2 surface for adsorption of organic dye having pyridyl group as anchoring unit, J. Phys. Chem. C 117(2013) 16364-16370. |
| [22] | Y. Ooyama, S. Inoue, T. Nagano, et al., Dye-sensitized solar cells based on donoracceptor p-conjugated fluorescent dyes with a pyridine ring as an electronwithdrawing anchoring group, Angew. Chem. Int. Ed. 50(2011) 7429-7433. |
| [23] | D.B. Kunag, S. Ito, B. Wenger, et al., High molar extinction coefficient heteroleptic ruthenium complexes for thin film dye-sensitized solar cells, J. Am. Chem. Soc. 128(2006) 4146-4154. |
| [24] | G.D. Sharma, S.P. Singh, R. Kurchania, R.J. Ball, Cosensitization of dye sensitized solar cells with a thiocyanate free Ru dye and a metal free dye containing thienylfluorene conjugation, RSC Adv. 3(2013) 6036-6043. |
| [25] | C.Y. Lin, C.F. Lo, L.Y. Luo, et al., Design and characterization of novel porphyrins with oligo(phenylethylnyl) links of varied length for dye-sensitized solar cells:synthesis and optical, electrochemical, and photovoltaic investigation, J. Phys. Chem. C 113(2009) 755-764. |
| [26] | C.M. Cardona, W. Li, A.E. Kaifer, D. Stockdale, G.C. Bazan, Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications, Adv. Mater. 23(2011) 2367-2371. |
| [27] | G. Boschloo, L. Häggman, A. Hagfeldt, Quantification of the effect of 4-tertbutylpyridine addition to I-/I3- redox electrolytes in dye-sensitized nanostructured TiO2 solar cells, J. Phys. Chem. B 110(2006) 13144-13150. |
| [28] | K.R.J. Thomas, Y.C. Hsu, J.T. Lin, et al., 2,3-disubstituted thiophene-based organic dyes for solar cells, Chem. Mater. 20(2008) 1830-1840. |
| [29] | H. Irie, Y. Watanabe, K. Hashimoto, Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders, J. Phys. Chem. B 107(2003) 5483-5486. |
| [30] | L.Q. Jing, X.J. Sun, J. Shang, et al., Review of surface photovoltage spectra of nanosized semiconductor and its applications in heterogeneous photocatalysis, Sol. Energy Mater. Sol. Cells 79(2003) 133-151. |
| [31] | G.D. Sharma, G.E. Zervaki, P.A. Angaridis, et al., Stepwise co-sensitization as a useful tool for enhancement of power conversion efficiency of dye-sensitized solar cells:the case of an unsymmetrical porphyrin dyad and a metal-free organic dye, Org. Electron. 15(2014) 1324-1337. |
| [32] | X. Jiang, T. Marinado, E. Gabrielsson, et al., Structural modification of organic dyes for efficient coadsorbent-free dye-sensitized solar cells, J. Phys. Chem. C 114(2010) 2799-2805. |
| [33] | P. Salvatori, G. Marotta, A. Cinti, et al., Supramolecular interactions of chenodeoxycholic acid increase the efficiency of dye-sensitized solar cells based on a cobalt electrolyte, J. Phys. Chem. C 117(2013) 3874-3887. |
| [34] | G.D. Sharma, S.P. Singh, P. Nagarjuna, et al., Efficient dye-sensitized solar cells based on cosensitized metal free organic dyes with complementary absorption spectra, J. Renew. Sustain. Energy 5(2013) 043107. |
| [35] | K. Mukherjee, T.H. Teng, R. Jose, S. Ramakrishna, Electron transport in electrospun TiO2 nanofiber dye-sensitized solar cells, Appl. Phys. Lett. 95(2009) 012101. |
| [36] | J. Bisquert, Theory of the impedance of electron diffusion and recombination in a thin layer, J. Phys. Chem. B 106(2002) 325-333. |
| [37] | J. Bisquert, Chemical capacitance of nanostructured semiconductors:its origin and significance for nanocomposite solar cells, Phys. Chem. Chem. Phys. 5(2003) 5360-5364. |
| [38] | A. Zaban, A. Meier, B.A. Gregg, Electric potential distribution and short-range screening in nanoporous TiO2 electrodes, J. Phys. Chem. B 101(1997) 7985-7990. |
| [39] | S. Ito, P. Liska, P. Comte, et al., Control of dark current in photoelectrochemical(TiO2/I-/I3-) and dye-sensitized solar cells, Chem. Commun. 34(2005) 4351-4353. |
| [40] | J. Bisquert, A. Zaban, M. Greenshtein, I. Mora-Seró, Determination of rate constants for charge transfer and the distribution of semiconductor and electrolyte electronic energy levels in dye-sensitized solar cells by open-circuit photovoltage decay method, J. Am. Chem. Soc. 126(2004) 13550-13559. |
| [41] | K. Fan, W. Zhang, T.Y. Peng, J.N. Chen, F. Yang, Application of TiO2 fusiform nanorods for dye-sensitized solar cells with significantly improved efficiency, J. Phys. Chem. C 115(2011) 17213-17219. |
| [42] | H. Yua, S.Q. Zhang, H.J. Zhao, G. Will, P. Liu, An efficient and low-cost TiO2 compact layer for performance improvement of dye-sensitized solar cells, Electrochim. Acta 54(2009) 1319-1324. |
| [43] | A. Zaban, M. Greenshtein, J. Bisquert, Determination of the electron lifetime in nanocrystalline dye solar cells by open-circuit voltage decay measurements, ChemPhysChem 4(2003) 859-864. |
 2016, Vol.27
2016, Vol.27