b Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Nitriles are versatile organic precursors in the synthesis of amines,amides,aldehydes,carboxylic acids,esters,and ketones, which are also key raw materials for agricultural chemicals,dyes, pharmaceuticals,and functional materials [1]. Furthermore,the cyano group is present in numerous bioactive molecules [2]. Two of the most classic methods for the preparation of aliphatic nitriles and aromatic nitriles are,respectively,the nucleophilic substitution reaction of alkyl halides with highly toxic inorganic cyanides [3] and the Sandmeyer reactions [4]. However,the most convenient and attractive method for the synthesis of nitriles is the dehydration of aldoximes,owing to their easy availability and avoiding the use of highly toxic inorganic cyanides. To date,lots of methods have been developed to effect the conversion of aldoximes to nitriles [5]. However,some of these methods suffer from the use of moisture-sensitive,highly expensive,and toxic or hazardous reagents,and some of them suffer from tedious work-up procedure. Therefore the search for more convenient and effective methods for dehydration of aldoximes is still desirable.
Perfluoroalkanosulfonyl fluoride (RfSO2F,such as n-C4F9SO2F and n-C8F17SO2F) is a kind of commercially available and cost effective reagent. RfSO2F is non-toxic and moisture-tolerant. In RfSO2F-induced reactions,the by-product is water-soluble perfluoroalkanesulfonic acid anion,which makes their work-up procedure very easy to handle; on the other hand,perfluoroalkanesulfonic acid salt RfSO3-M+ is also a kind of highly valued surfactant,and if needed,perfluoroalkanesulfonic acid can be easily isolated from the aqueous layer by adjusting its pH value with aqueous hydrochloric acid solution after work-up procedure. On the basis of the excellent leaving ability of perfluorosulfonate anion (in comparison with p-toluenesulfonate anion and methanesulfonate anion) [6] and the ease of reaction of perfluoroalkanosulfonyl fluoride with alcohol in the presence of a base,RfSO2F has been developed and used as an excellent hydroxyl groupactivating reagent for fluorination of alcohols [7],for cyclodehydration of chiral vicinal diols [8],N-acylamino alcohols,β-hydroxy sulfonamides and β-hydroxy thioamides [9]. Very recently, perfluoroalkanosulfonyl fluoride-induced Beckmann rearrangement of α,β-unsaturated ketoximes in alkaline media leading to the smooth formation of enamides was reported by us [10]. However, to the best of our knowledge,the reaction of perfluoroalkanosulfonyl fluoride with aldoximes has not been investigated yet. Herein,we wish to report the results of dehydration of aldoximes to nitriles induced by perfluoroalkanosulfonyl fluoride in alkaline media (Scheme 1).
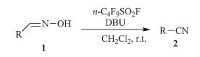
|
Download:
|
| Scheme 1.n-C4F9SO2F-induced dehydration of aldoximes to nitriles. | |
The general procedure of n-C4F9SO2F-induced dehydration of a variety of aldoximes to nitriles: at room temperature,n-C4F9SO2F (20.0 mmol,2.0 equiv.) was slowly dropped via syringe into a solution of substrate 1a (10.0 mmol) and 1,8-diazabicyclo[ 5.4.0]undec-7-ene (DBU,50.0 mmol,5.0 equiv.) in CH2Cl2 (20 mL). The resulting solution was stirred at this temperature for 10 min. Evaporation under vacuum to remove volatile components offered residue which was purified through silica gel column chromatography (eluted with a mixture of petroleum ether and ethyl acetate),generating nitrile 2a in 95% yield. Colorless solid,mp 143-146 ℃. 1H NMR (400 MHz,CDCl3): δ 8.35 (d,2H,J = 8.0 Hz),7.88 (d,2H,J = 8.0 Hz); 13C NMR (101 MHz, CDCl3): δ 150.01,133.43,124.26,118.31,116.74.
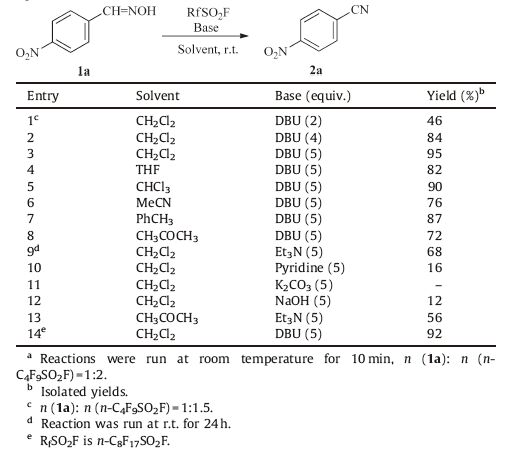
3. Results and discussionTo optimize the reaction conditions,p-nitrobenzaldoxime (1a) was selected as a model substrate under various reaction conditions (Table 1). First,using dichloromethane as solvent and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as base,we explored the effect of the molar ratio of n (1a): n (n-C4F9SO2F): n (DBU) on dehydration at room temperature. When the molar ratio of 1a/n- C4F9SO2F/DBU is 1:1.5:2,the yield of 2a is 46% with 40% of 1a recovered after 10 min (Table 1,entry 1); the longer reaction time did not improve the yield of 2a. When the ratio was increased to 1:2:5,the best result was obtained with the formation of 2a in 95% yield (entry 3). When the reaction was carried out at 0 ℃,a similar result was obtained; however when the reaction was run at reflux, some unidentified impurities formed. Solvent effects on dehydration were investigated (entries 4-8). Among screened solvents of acetonitrile,toluene,chloroform,dichloromethane,acetone and tetrahydrofuran,dichloromethane was found to be the best choice, and the use of the other solvents resulted in lower yields of 2a in the range of 72-90%. Several organic and inorganic bases,such as pyridine,triethylamine,DBU,K2CO3,and NaOH were further tested,and the results showed that the use of DBU as base offered the best yield (entries 9-13). Finally both n-C4F9SO2F and n-C8F17SO2F showed imilar activity (entry 14). Therefore,the optimized reaction conditions for the dehydration of 1a are: CH2Cl2 as solvent,DBU as base,1a: n-C4F9SO2F: DBU = 1:2:5,room temperature,and reaction time of 10 min.
|
|
Table 1 Optimization of reaction conditions.a |
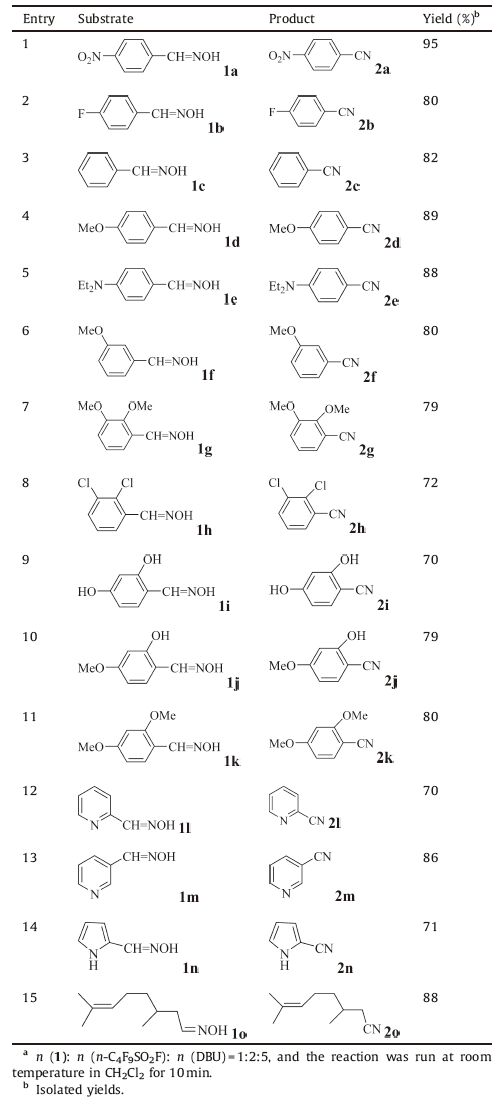
With the optimized reaction conditions in hand,a variety of aldoximes were subjected to the n-C4F9SO2F/DBU system for dehydration to explore the scope and limitations of the reaction. The results are presented in Table 2. All the aldoximes were smoothly converted to the corresponding nitriles in 70%-95% yields. The dehydration of pyridine-3-carbaldehyde oxime provided 3-cyanopyridine in 86% yield (entry 13). For comparison,the dehydration of pyridine-3-carbaldehyde oxime with AlCl3⋅6H2O/ KI/H2O/CH3CN system [11] and triphenylphosphine/I2 system [12] provided 3-cyanoprridine in 69% yield (after refluxing at 80 ℃ for 6 h) and in 84% yield (at room temperature for 4 h),respectively. The method is applicable to aromatic,aliphatic,and heteroaryl aldoximes and is tolerant to a wide range of functional groups such as methoxy,hydroxyl,halide,and diethylamino groups. Electrondonating or electron-withdrawing group on the aromatic ring has little effect on the yield of the product. The work-up procedure is simple,just needing evaporation under vacuum to remove volatile components and subsequent purification of residue through silica gel column chromatography with a mixture of petroleum ether and ethyl acetate as eluent. All the products were confirmed by 1 HNMR and 13C NMR,IR and MS spectra,and the data were identical with literature records.
|
|
Table 2 n-C4F9SO2F-Induced dehydration of a variety of aldoximes to nitriles.a |
In conclusion,an efficient method was developed for the dehydration of aldoximes in alkaline media and nitrile products were obtained in 70%-95% yields. Mild reaction conditions,simple work-up procedure,and high yields are advantages of the presented method.
AcknowledgmentThe authors thank the National Natural Science Foundation of China (No. 21362022) for financial support.
| [1] | (a) J.S. Miller, L. Manson, Designer magnets containing cyanides and nitriles, Acc. Chem. Res. 34(2001) 563-570;(b) M.A. Cohen, J. Sawden, N.J. Turner, Selective hydrolysis of nitriles under mild conditions by an enzyme, Tetrahedron Lett. 31(1990) 7223-7226. |
| [2] | F.F. Fleming, L. Yao, P.C. Ravikumar, B.C. Shook, Nitrile-containing pharmaceuticals:efficacious roles of the nitrile pharmacophore, J. Med. Chem. 53(2010) 7902-7917. |
| [3] | M.J. Kiefel, Synthesis:carbon with one heteroatom attached by a single bond, in:A.R. Katrizky, O. Meth-Cohn, C.W. Rees(Eds.), Comprehensive Organic Functional Group Transformations, Pergamon, Cambridge, UK, 1995, pp. 641-676. |
| [4] | T. Sandmeyer, Ueber die ersetzung der amidgruppe durch chlor in den aromatischen substanzen, Ber. Dtsch. Chem. Ges. 17(1884) 1633-1635. |
| [5] | (a) B. Movassagh, S. Shokri, Potassium fluoride doped on alumina:an efficient catalyst for conversion of aldoximes into nitriles, Synth. Commun. 35(2005) 887-890;(b) J.A. Campbell, G. McDougald, H. McNab, L.V.C. Rees, R.G. Tyas, Laboratoryscale synthesis of nitriles by catalysed dehydration of amides and oximes under flash vacuum pyrolysis(FVP) conditions, Synthesis 39(2007) 3179-3184;(c) J.K. Augustine, R. Kumar, A. Bombrun, A.B. Mandal, An efficient catalytic method for the Beckmann rearrangement of ketoximes to amides and aldoximes to nitriles mediated by propylphosphonic anhydride(T3P®), Tetrahedron Lett. 52(2011) 1074-1077;(d) Z. Moussa, S.A. Ahmed, A.S. ElDouhaibi, S.Y. Al-Raqa, NMR Studies and electrophilic properties of triphenylphosphine-trifluoromethanesulfonic anhydride; a remarkable dehydrating reagent system for the conversion of aldoximes to nitriles, Tetrahedron Lett. 51(2010) 1826-1831;(e) D. Saha, A. Saha, B.C. Ranu, Ionic liquid-promoted dehydration of aldoximes:a convenient access to aromatic, heteroaromatic and aliphatic nitriles, Tetrahedron Lett. 50(2009) 6088-6091;(f) L.D.S. Yadav, V.P. Srivastava, R. Patel, Bromodimethylsulfonium bromide(BDMS):a useful reagent for conversion of aldoximes and primary amides to nitriles, Tetrahedron Lett. 50(2009) 5532-5535;(g) R.G. Kalkhambkar, S.D. Bunge, K.K. Laali, Reaction of triflyl-imidazole with aldoximes:facile synthesis of nitriles and formation of novel aldoxime-bis(Ntriflyl)-imidazole adducts, Tetrahedron Lett. 52(2011) 5184-5187;(h) L. Yu, H. Li, X. Zhang, et al., Organoselenium-catalyzed mild dehydration of aldoximes:an unexpected practical method for organonitrile synthesis, Org. Lett. 16(2014) 1346-1349;(i) Y. Song, D. Shen, Q. Zhang, B. Chen, G. Xu, Ac2O/K2CO3/DMSO:an efficient and practical reagent system for the synthesis of nitriles from aldoximes, Tetrahedron Lett. 55(2014) 639-641(and references cited therein). |
| [6] | R.L. Hansen, Perfluoroalkanesulfonate esters as alkylating agents, J. Org. Chem. 29(1965) 4322-4324. |
| [7] | H. Vorbruggen, The Conversion of primary or secondary alcohols with nonaflyl fluoride into their corresponding inverted fluorides, Synthesis 40(2008) 1165-1174. |
| [8] | Z. Yan, J. Wang, W. Tian, A concise total synthesis of(-)-dehydroclausenamide utilizing the novel formation of cis-epoxide as the key step, Tetrahedron Lett. 44(2003) 9383-9384. |
| [9] | Z. Yan, C. Guan, Z. Yu, W. Tian, Fluoroalkanosulfonyl fluorides-mediated cyclodehydration of β-hydroxy sulfonamides and β-hydroxy thioamides to the corresponding aziridines and thiazolines, Tetrahedron Lett. 54(2013) 5788-5790. |
| [10] | Z. Yan, Y. Xu, W. Tian, A new and concise way to enamides by fluoroalkanosulfonyl fluoride mediated Beckmann rearrangement of α,β-unsaturated ketoximes, Tetrahedron Lett. 55(2014) 7186-7189. |
| [11] | M. Boruah, D. Konvar, AlCl3·3H2O/KI/H2O/CH3CN:a new alternate system for dehydration of oximes and amides in hydrated media, J. Org. Chem. 67(2002) 7138-7139. |
| [12] | A.V. Narsaiah, D. Sreenu, K. Nagaiah, Triphenylphosphine-I2:an efficient reagent system for the synthesis of nitriles from aldoximes, Synth. Commun. 36(2006) 137-140. |
 2016, Vol.27
2016, Vol.27