b Key Laboratory of Organo-pharmaceutical Chemistry of Jiangxi Province, Gannan Normal University, Ganzhou 341000, China
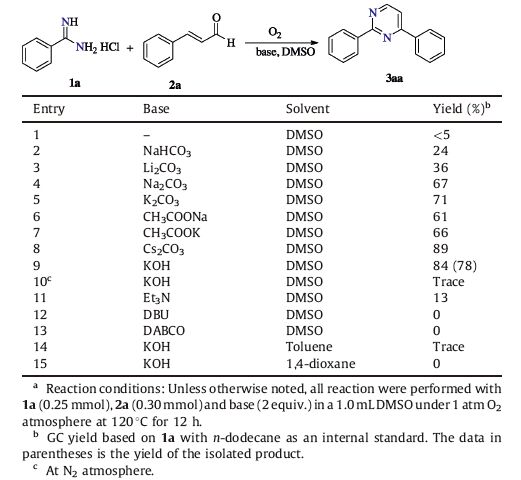
Pyrimidines are very important heterocycles featuring prominently in the synthesis of pharmaceuticals,agrochemicals,and functional materials as well [1]. Particularly,they represent an increasing valuable goal because of their large range of applications as anti-plasmodial agents,antimalarial agents,cytotoxic inhibitors and photophysical materials [2]. While there exist various synthetic methods for the useful heterocyles via cyclization- oxidation processes,amidines are frequently used to prepare multiple-nitrogen-containing heterocycles because of their innate structural advantages [3]. Numbers of synthetic routes have also been developed for the synthesis of pyrimidines through the cascade condensation cyclization-oxidation of amidines with 1,3- dicarbonyl derivatives or a,β-unsaturated ketones [4]. Bagley and co-workers successfully demonstrated a tandem oxidation/heterocyclocondensation of a propargylic alcohol and benzamidine for the synthesis of pyrimidines using BaMnO4 under microwave irradiation [5] (Scheme 1,a). Lin developed a Cu(OTf)2-catalyzed tandem reaction of propargylic alcohols with amidine,providing a general approach to pyrimindines [6] (Scheme 1,b). Recently,Guirado published a chloroform elimination of 2,4-diaryl-6- trichloromethyl-1,6-dihydropyrimidines to 2,4-diarylpyrimidines starting from 2,2,2-trichloroethylidene-acetophenones and benzamidines [7] (Scheme 1,c). The condensation of propargylamine and benzamidine to give pyrimidine was also developed by Chen and co-workers [8] (Scheme 1,d). However,most of the mentioned methods suffered from harsh reaction conditions (special reaction medium,microwave irradiation,transition-metals) and the use of relatively unavailable starting materials. Hence,the development of a simple and efficient procedure for acquisition of pyrimidines from easily available starting materials under mild conditions continues to attract the interest of organic chemists due to their remarkable application value.
|
Download:
|
| Scheme 1.General approaches for the synthesis of pyrimidine derivatives. | |
Direct C-H diamination represents one of the most powerful synthetic protocols for the construction of N-heterocycles,avoiding the pre-installation of transformable functional groups and possessing atom economy and environmental sustainability [9]. Many elegant methods involving transition-metal catalization and the use of overstoichiometric amounts of oxidants have been dominated [10]. For green and sustainable chemistry,molecular oxygen is considered as an ideal oxidant due to its natural,inexpensive,and environmentally friendly characteristics,and therefore shows attractive academic and industrial prospects [11]. In the past few years,our group developed serials of C-H dioxygen activation reactions using molecular oxygen as the terminal oxidant [12],we therefore think about employment of the molecular oxygen as the oxidant for the pyrimidine synthesis. Herein,we further report a base mediated direct C-H amination of amidines and cinnamaldehydes to afford polysubstitutedpyrimidines usingmolecular oxygen as sole oxidant (Scheme 1,e).
2. ExperimentalAll of the reagents were used directly as obtained commercially. Column chromatography was performed with silica gel (200-300 mesh) and analytical TLC on silica GF254. Melting points were measured using a melting point instrument and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a 400 MHz NMR spectrometer. IR spectra were obtained with an infrared spectrometer on either potassium bromide pellets or liquid films between two potassium bromide pellets. GC-MS data were obtained using electron ionization. HRMS was carried out on a high-resolution mass spectrometer (LCMS-IT-TOF).
2.1. General procedure for 3aaA mixture of benzamidine hydrochloride 1a (0.25 mmol),cinnamaldehyde 2a (0.30 mol) and KOH (0.50 mmol,2 equiv.) was stirred in DMSO (1.0 mL) under 1 atm O2 atmosphere at 120 ℃ for 12 h. After completion of the reaction (monitored by TLC),water (10 mL) was added to the reaction mixture,and the resulting mixture was extracted with ethyl acetate. The combined organic layers were then dried over MgSO4,filtered,and then concentrated in vacuo. The residue was purified by flash chromatography on silica gel to give the desired product 3aa as a white solid (using the mixture of petroleum ether and ethyl acetate as eluents). Yield: 0.045 g (78%),mp 63-65 ℃; IR (KBr,cm-1 ): n 3064,1563,1422,1383,1182,1068,1030,747,691; 1H NMR (400 Hz,CDCl3): d 8.78-8.77 (m,1H),8.59-8.58 (m,2H),8.19-8.18 (m,2H),7.52-7.49 (m,7H); 13C NMR (100 Hz,CDCl3): δ 164.59,163.87,157.86,137.91,136.96,131.01,130.78,128.97,128.59,128.36,127.24,114.54; MS (EI,70 eV) m/z: 232.13,129.11,116.16,102.08; HRMS (ESI) Calcd. C16H13N2 [M+H]+: 233.1073,found: 233.1070. The data of 3ab-3la were available in Supporting information.
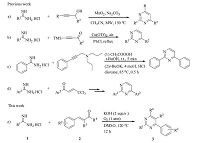
3. Results and discussionWe initiated our study by using benzamidine hydrochloride 1a and cinnamaldehyde 2a as model substrates under various conditions and the results are summarized in Table 1. In the absence of a base,the reaction between 1a and 2a gave a low yield of 2,4-diphenylpyrimidine 3aa (Table 1,entry 1). When this reaction was performed in the presence of bases,such as NaHCO3,Li2CO3,Na2CO3,K2CO3,CH3COONa,CH3COOK,CS2CO3 and KOH,the yield of 3aa increased (Table 1,entries 2-9). Especially,the reaction performed with CS2CO3 gave the best result (89% GC yield) (Table 1,entry 9). Trace of 3aa was detected in N2 atmosphere (Table 1,entry 10). As a result,both the base and O2 were found to be indispensable. Organic bases such as Et3N,DBU,and DABCO exhibited poor results (Table 1,entries 11-13). Considering the costs of Cs2CO3 and KOH,we used KOH to further optimize this transformation. Screening of different solvents revealed that DMSO was the best solvent for this process. Trace or No 3aa were detected by GC-MS when using toluene or 1,4-dioxane as solvents (Table 1,entries 14-15). Therefore,the best conditions for this transformation involved 2 equiv. KOH,in DMSO at 120 ℃ for 12 h.
|
|
Table 1 Selected optimization studies.a |
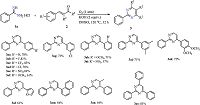
Under the established conditions,benzamidine hydrochloride 1a and various cinnamaldehydes were explored as substrates,and the results are summarized in Fig. 1. A series of para-substituted cinnamaldehydes,including some with electron-donating groups and some with electron-withdrawing groups (R0 = F,Cl,CF3,NO2),proceeded smoothly and afforded the desired pyrimidine products in high yields (3ab-3af). Other substituted cinnamaldehydes,such as meta-,and ortho-substituted substrates,could also provide the desired product (3ag-3ai). These results showed that this transformation was tolerant towards the electronic and steric effects of the aromatic ring. In addition,polysubstituted cinnamaldehydes also gave satisfied results (3aj-3ak). (E)-3-(Furan-2- yl)acrylaldehyde (2l) also produced the desired products in fair yields. To our delight,(E)-3-(naphthalen-1-yl)acrylaldehyde could give the corresponding product in 64% yield (3am). Interestingly,the transformation of (E)-2-methyl-3-phenylacrylaldehyde proceeded efficiently under the optimized conditions affording the desired product (3an) in a moderate yield. In particular,(E)- chalcone delivered the products (3ao) in 83% yield,which might be attributed to the formation of macro p-conjugation bonds,promoting the amination process.
|
Download:
|
| Fig. 1.Scope of cinnamaldehydes substrates. Reaction conditions: 1a (0.25 mmol),2 (0.30 mmol) and KOH (2 equiv.) in a 1.0 mL DMSO under 1 atm O2 atmosphere at 120 ℃ for 12 h. Isolated yield based on 1a. | |
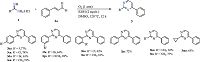
To further expand the substrate scope,the substrates benzamidines were investigated (Fig. 2). To our satisfaction,both electron-donating and electron-withdrawing groups attached benzamidines were all suitable for this protocol,and provided the corresponding products in 61%-77% yields (3ba-3ia). Gratifyingly,72% yield of 3ja was observed when isonicotinamidine was used in thisamination reaction. Non-aromaticamidineswere also tolerated well,delivering the desired products (3ka-3ma) in moderate to good yields. It is worth noting that an important pharmaceutical intermediates (3la) [13] was successful obtained from guanidine and cinnamaldehyde. The product provides an opportunity for further selective function at amino group in pharmaceuticals/ agrochemicals design.
|
Download:
|
| Fig. 2.Scope of amidines substrates. Reaction conditions: 1 (0.25 mmol),2a (0.30 mmol) and KOH (2 equiv.) in a 1.0 mL DMSO under 1 atm O2 atmosphere at 120 ℃ for 12 h. Isolated yield based on 1. | |
Based on the above-mentioned experimental observations and previous report [14],we proposed the mechanism for the reaction shown in Scheme 2. This mechanism involves (1) deprotonation of benzimidamide under strong base conditions to give intermediate A; (2) the nucleophilic addition of intermediate A to 2a could deliver Intermediate B which then rearranged to intermediate C; (3) a further nucleophilic addition process occurred to give intermediate D; (4) intermediate D was further underwent the oxidation process and afforded the desired product 3aa.
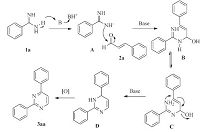
|
Download:
|
| Scheme 2.Proposed mechanism. | |
In conclusion,we have developed the facile synthesis of pyrimidine derivatives by a simple based mediated direct C-H amination of amidines and cinnamaldehydes to afford polysubstituted pyrimidines using molecular oxygen as green oxidant. This greener synthetic methodology provides a straightforward approach for the synthesis of a variety of pyrimidine derivatives,avoiding the used of transition-metals and harmful oxidants.
AcknowledgmentsWe are grateful to the China Postdoctoral Science Foundation Funded Project (No. 2014M562165),Jiangxi Natural Science Foundation (Nos. 20133BCB24011,20141BBG70070 and 20151BAB203011) and the Science Foundation of Jiangxi Provincial Department of Education (No. Gjj4669) for support of this research.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.09.012.
| [1] | M.W. Martin, J. Newcomb, J.J. Nunes, et al., Novel 2-aminopyrimidine carbamates as potent and orally active inhibitors of lck:synthesis, SAR, and in vivo antiinflammatory activity, J. Med. Chem. 49(2006) 4981-4991. |
| [2] | (a) S. Lee, D. Lim, E. Lee, et al., Discovery of carbohybrid-based 2-aminopyrimidine analogues as a new class of rapid-acting antimalarial agents using imagebased cytological profiling assay, J. Med. Chem. 57(2014) 7425-7434;(b) F. Arioli, S. Borrelli, F. Colombo, et al., N-[2-Methyl-5-(triazol-1-yl)phenyl]-pyrimidin-2-amine as a scaffold for the synthesis of inhibitors of Bcr-Abl, ChemMedChem 6(2011) 2009-2018. |
| [3] | (a) H. Huang, W. Guo, W. Wu, et al., Copper-catalyzed oxidative C(sp3)-H functionalization for facile synthesis of 1,2,4-triazoles and 1,3,5-trizaines from amidines, Org. Lett. 17(2015) 2894-2897;(b) W. Guo, K. Huang, F. Ji, et al., A facile approach to synthesize 3,5-disubstituted-1,2,4-oxadiazoles via copper-catalyzed-cascade annulations of amidines and methylarenes, Chem. Commun. 51(2015) 8857-8860. |
| [4] | K.S. Vadagaonkar, H.P. Kalmode, S. Prakash, et al., Greener[3+3] tandem annulation-oxidation approach towards the synthesis of substituted pyrimidines, New J. Chem. 39(2015) 3639-3645. |
| [5] | M.C. Bagley, Z. Lin, S.J.A. Pope, Barium manganate in microwave-assisted oxidation reactions:synthesis of solvatochromic 2,4,6-triarylpyrimidines, Tetrahedron Lett. 50(2009) 6818-6823. |
| [6] | M. Lin, Q.Z. Chen, Y. Zhu, Copper(Ⅱ)-catalyzed synthesis of pyrimidines from propargylic alcohols and amidine:a propargylation-cyclization-oxidation tandem reaction, Synlett 8(2011) 1179-1183. |
| [7] | A. Guirado, E. Alarcón, Y. Vicente, et al., A new improved method for the synthesis of 2,4-diarylpyrimidines starting from 2,2,2-trichloroethylideneacetophenones, Tetrahedron Lett. 54(2013) 5115-5117. |
| [8] | J. Chen, R. Properzi, D.P. Uccello, et al., One-pot oxidation and rearrangement of propargylamines and in situ pyrazole synthesis, Org. Lett. 16(2014) 4146-4149. |
| [9] | R.M. de Figueiredo, Transition-metal-catalyzed diamination of olefins, Angew. Chem. Int. Ed. 48(2009) 1190-1193. |
| [10] | (a) Y. Zhu, R.G. Cornwal, H. Du, et al., Catalytic diamination of olefins via N-N bond activation, Acc. Chem. Res. 47(2014) 3665-3678;(b) D. Chen, H.J. Mo, D.B. Chen, Direct C-H amination for indole synthesis from N-Ts-2-styrylaniline derivatives catalyzed by copper salt, Chin. Chem. Lett. 26(2015) 969-972;(c) Q. Cai, M.C. Liu, B.M. Mao, et al., Direct one-pot synthesis of zolimidine pharmaceutical drug and imidazo[1,2-a]pyridine derivatives via I2/CuO-promoted tandem strategy, Chin. Chem. Lett. 26(2015) 881-884. |
| [11] | Z. Shi, C. Zhang, C. Tang, et al., Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant, Chem. Soc. Rev. 41(2012) 3381-3430. |
| [12] | W. Wu, H. Jiang, Palladium-catalyzed oxidation of unsaturated hydrocarbons using molecular oxygen, Acc. Chem. Res. 45(2012) 1736-1748. |
| [13] | R.J. Altenbach, R.M. Adair, B.M. Bettencourt, et al., Structure-activity studies on a series of a 2-aminopyridimidine-containing histamine H4 receptor ligands, J. Med. Chem. 51(2008) 6571-6580. |
| [14] | L. Li, Y.L. Zhao, Q. Wang, Base-promoted oxidative C-H functionalization of α-amino carbonyl compounds under mild metal-free conditions:using molecular oxygen as the oxidant, Org. Lett. 17(2015) 370-373. |
 2016, Vol.27
2016, Vol.27