Carbon-carbon coupling reactions are among the most powerful synthetic tools used in the synthesis of complex molecules such as natural products. Mizoroki and Heck independently reported the first carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle. It involved the coupling of aryl or vinyl halides and activated alkenes in the presence of a base [1, 2]. Conversely,there has long been an increasing interest in the transition metal-catalyzed functionalization of relatively unactivated carbon-hydrogen (C-H) bonds for the new C-C,C- O,or C-S bond formation. This approach is straightforward,environmentally friendly and avoids the use of protective/leaving groups,thus significantly shortening the synthetic routes [3, 4]. The C-H activation finds applications in the synthesis of natural products,functional materials,and pharmaceuticals. Some recent examples include the palladium-catalyzed enantioselective C-H arylation in the synthesis of P-stereogenic compounds [5],palladium(II)-catalyzed directed trifluoromethylthiolation of unactivated C(sp3)-H bonds [10],cobalt catalyzed low temperature CH alkenylations with alkenyl acetates,phosphates,carbonates or carbamates [6] and photocatalytic CH activation by hydrogen-atom transfer [7]. Moreover,direct C-H amination for indole synthesis from N-Ts-2-styrylaniline derivatives catalyzed by copper salt [8],multi-substituted arenes via palladium catalyzed C-H halogenation [9],efficient synthesis of 2-arylquinazolines via copper-catalyzed dual oxidative benzylic C-H aminations of methylarenes [10] have also been reported.
Thiourea derivatives have long been employed as organocatalysts for various organic transformations. These ligands have also been used in palladium catalyzed Heck and Suzuki-Miyaura cross coupling reactions [11, 12]. N,N0-Monosubstituted acyclic thiourea and 1-aroyl-3-arylthiourea ligands have been utilized as highly active phosphine-free catalysts for palladium catalyzed Heck reactions of aryl iodides or bromides with styrenes and acrylates. The 1-aroyl-3-arylthiourea ligands giving best results included the simple 1-benzoyl-3-phenylthiourea and 1-(4-bromobenzoyl)-3- (4-bromophenyl)thiourea with bulky bromo-substituents [13].
Substituted triazolo[3, 4-b][1, 3, 4]thiadiazoles are the key structural motifs of numerous compounds with activity relevant to biological and medicinal chemistry [14, 15]. Therefore,there is an unrelenting strong requirement for developing new strategies for the synthesis of these heterocycles.
The significance and utilization of the theories mentioned above together with the availability of our library of diverse 1-acyl- 3-arylthiourea derivatives [16],encouraged us to explore the C-H activated coupling of 6-aryl [1, 2, 4]triazolo [3, 4-b][1, 3, 4]thiadiazole with different aryl iodides in the presence of 1-(2-naphthoyl)- 3-(4-bromophenyl)thiourea.
2. ExperimentalMelting points were recorded using a digital Gallenkamp (SANYO) model MPD.BM 3.5 apparatus and are uncorrected. 1H NMR and 13C NMR spectra were determined at 300 MHz and 75 MHz,respectively,using a Bruker AM-300 spectrophotometer. FTIR spectra were recorded on a Bio-Rad-Excalibur Series Mode FTS 3000 MX spectrophotometer. Mass Spectra (EI,70 eV) on a GC-MS,Agilent Technologies 6890N and an inert mass selective detector 5973 mass spectrometer Technologies and elemental analyses were conducted using a LECO-183 CHNS analyzer. Thin layer chromatography (TLC) was conducted on 0.25 mm silica gel plates (60 F254,Merck).
2.1. Synthesis of 6-aryl[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazoles(4a-o) 4-Amino-4H-1,2,4-triazole-3-thiole (1 equiv.) (3) was taken in a two-necked round bottom flask equipped with magnetic stirrer and suitable benzoic acid (1 equiv.) was added followed by the dropwise addition of the POCl3 (10 mL). The reaction mixture was refluxed at 180 ℃ for 10 h. On completion,the reaction mixture was cooled and neutralized with NaHCO3 and the solids obtained were filtered and recrystallized from water and ethanol (8:2) to afford products (4a-o) in 69%-81% yields.
2.2. Synthesis of 3-aryl-6-phenyl[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazoles(5a-o) Pd(OAc)2 (5 mol%) and thiourea ligand (3 mol%) were added to 15 mL of DMA (15 mL) and stirred well under argon. Then,CaO (1.5 equiv.) followed by that of corresponding triazolo-thiadiazole (4a-o) (1.0 equiv.) were added to the reaction mixture and refluxed at 140 ℃ for 1 h. After cooling to room temperature 4- iodoaniline derivative (1.0 equiv.) was added and the mixture refluxed for 15-20 h. The reaction progress was monitored by TLC analysis using a solvent system (methanol:chloroform = 0.5:9.5) or (ethyl acetate: n-hexane = 6:4). On completion,the reaction mixture was poured into 50 mL of water. The water layer was extracted twice with 25 mL of DCM,dried (anhydrous sodium sulphate) filtered and concentrated. The products were purified by recrystallization or by column extraction using n-hexane/ethyl acetate (3:1) to yield the 3,6-diphenyl-[1, 2, 4]triazolo[3,4- b][1, 3, 4]thiadiazoles (5a-o).
3,6-Diphenyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5a): Yellow solid,Rf = 0.67 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1 ): 1605 (N=O str),1514 (C=N str),1494 (Ar,C=C str),1185 (Ar,C-C str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): d 7.52-7.45 (m,5H,J = 9.0 Hz,Ar-H),7.39-7.42 (m,5H,J = 9.0 Hz,Ar- H),13C NMR (75.5 MHz,DMSO-d6): δ 167.04,141.9,133.3 (C=N),131.9,131.9,130.4,130.05,129.9,129.7,128.6,127.8 (ArCs),Anal. Calcd. for C15H10N4S: C,64.73; H,3.62; N,20.13; S,11.52; found: C,64.71; H,3.64; N,20.13; S,11.52. GC-MS m/z: 278.0 (M+,100).
6-Phenyl-3-m-tolyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5b): Yellow solid,Rf = 0.66 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1): 2921 (Ar,C-H str),2851 (methyl,C-H str),1602 (nitro N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 7.70 (d,1H,J = 1.2 Hz),7.68-7.54 (m,1H,Ar-H),7.42-7.30 (m,2H,Ar-H),7.39- 7.42 (m,5H,J = 9 Hz,Ar-H),2.42 (s,3H,ArCH3). 13C NMR (75.5 MHz,DMSO-d6): δ 167.04,141.9,133.3 (C=N),131.9,131.9,130.4,130.05,129.9,129.7,128.6,127.8 (ArCs),21.58 (CH3),Anal. Calcd. for C16H14N4S: C,65.73; H,4.14; N,19.17; S,10.96; found: C,65.71; H,4.16; N,19.15; S,10.98. EI-MSm/z: 292.0 (M+,100).
6-Phenyl-3-p-tolyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5c): Yellow solid; Rf = 0.68 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1): 3064 (Ar,C-H str),2923 (methyl,C-H str),1517 (C=N str),1466 (Ar,C=C str),971 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 8.28 (d,2H,J = 7.5 Hz,Ar-H),8.10 (d,1H,J = 7.5 Hz,Ar-H) 7.90-7.24 (m,5H,J = 9 Hz,Ar-H),2.41 (s,3H,ArCH3). 13C NMR (75.5 MHz,DMSO-d6): δ 167.0,140.9,133.0 (C=N),132.9,131.7,130.1,130.0,129.8,129.6,128.5,127.7 (ArCs),21.= (CH3),Anal. Calcd. for C16H14N4S: C,65.73; H,4.14; N,19.16; S,10.97; found: C,65.71; H,4.16; N,19.15; S,10.98. EI-MS m/z: 292.0 (M+,100).
3-(4-Chlorophenyl)-6-phenyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5d): Light yellow solid; Rf = 0.66 (petroleum ether:ethyl acetate = 7:3) IR (KBr,cm-1): 2921 (Ar,C-H str),2851 (methyl,C-H str),1602 (nitro N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 7.73 (d,2H,J = 7.8 Hz,ArH),7.47 (d,2H,J = 7.8 Hz,ArH),7.39-7.42 (m,5H,J = 9 Hz,Ar-H). 13C NMR (75.5 MHz,DMSO-d6): δ 165.2,151.0,146.3 (C=N),143.9,131.9,129.5,128.3,127.8,124.6,123.5,114.5 (ArCs). Anal. Calcd. for C1sH9ClN4S: C,57.60; H,2.90; N,17.91; S,10.25; found: C,57.62; H,2.88; N,17.90; S,10.26. EI-MSm/z: 312.0,314.0 (M+,100).
3-(4-Nitrophenyl)-6-phenyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5e): Yellow solid; Rf = 0.56 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1): 2921 (Ar,C-H str),2851 (methyl,C-H str),1602 (nitro N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): d 7.36 (d,2H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar-H); 7.39- 7.42 (m,5H,J = 9 Hz,Ar-H). 13C NMR (75.5 MHz,DMSO-d6): d 166.2,153.0,146.4 (C=N),145.9,132.9,129.6,127.3,126.8,126.6,124.5,115.5 (ArCs); Anal. Calcd. for C1sH9N5O2S: C,=.72; H,2.81; N,21.66,S,9.92; found: C,=.70; H,2.83; N,21.65; S,9.93. EI-MS m/z: 323 (M+,100).
4-(6-Phenyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazol-3-yl)benzenamine (5f): Light brown; Rf = 0.62 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1): 3064 (Ar,C-H str),2923 (methyl,C-H str),1518 (C=N str),1465 (Ar,C=C str),971 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 8.03 (d,2H,J = 8.0 Hz,ArH),7.96 (d,2H,J = 7.5 Hz,ArH),7.66-6.72 (m,5H,J = 9 Hz,Ar-H),5.69 (s,2H,NH2). 13C NMR (75.5 MHz,DMSO-d6): δ 165.7,152.4,150.8 (C=N),146.3,132.7,129.5,129.0,127.1,129.1,113.6,112.3 (ArCs). Anal. Calcd. for C1sH11N5S: C,61.42; H,3.78; N,23.87; S,10.93; found: C,61.40; H,3.80; N,23.86; S,10.94. EI-MS m/z: 293 (M+,100).
6-Phenyl-3-(pyridin-4-yl)-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5g): Yellow solid; Rf = 0.66 (petroleum ether:ethyl acetate 7:3); IR (KBr,cm-1): 3080 (sp2 CH),1626 (C=N),1505 (C=C),1266 (N-N=C triazolo-thiadiazole),669 (C-S-C),1H NMR (DMSO-d6): d 8.84-8.25 (m,4H,Py),8.10-7.65 (m,5H,J = 9 Hz; Ar-H). 13C NMR (75.5 MHz,DMSO-d6): δ 167.5 150.7,143.7,(C=N),133.1 132.4,129.7,128.8,127.4,126.4,126.3,119.4,(ArCs). Anal. Calcd. for C14H9N5S,C,60.20; H,3.25; N,25.07; S,11.48; found: C,60.22; H,3.86; N,25.09; S,11.46. EI-MS m/z: 279 (M+,100).
3-(4-Chlorophenyl)-6-(4-nitrophenyl)-[1, 2, 4]triazolo[3,4- b][1, 3, 4]thiadiazole (5h): Yellow solid; Rf = 0.33 (petroleum ether:ethyl acetate 7:3); IR (KBr,cm1): 2921 (Ar C-H str),2851 (methyl C-H str),1602 (nitro N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 7.36 (d,1H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar- H); 7.73 (d,2H,J = 7.8 Hz,ArH),7.47 (d,2H,J = 7.8 Hz,ArH). 13C NMR (75.5 MHz,DMSO-d6): δ 166.5,149.1,143.5,(C=N),1425,139.3,137.1,136.2,133.7 130.4,129.2,122.4,(ArCs). Anal. Calcd. for C15H8ClN5O2S,C,50.36; H,2.25; N,19.57 S,8.96; found: C,50.34; H,2.27; N,19.56; S,8.97. EI-MS m/z: 357 (M+,100).
3-(4-Chlorophenyl-)-6-(4-chlorophenyl)-[1, 2, 4]triazolo[3,4- b][1, 3, 4]thiadiazole (5i): White solid; Rf = 0.68 (petroleum ether:- ethyl acetate 7:3); IR (KBr,cm-1 ): 3080 (sp2 CH),1626 (C=N),1505 (Ar,C=C),1266 (N-N=C triazolo-thiadiazole),669 (C-S-C),1H NMR (DMSO-d6): δ 7.73 (d,2H,J = 7.8 Hz,ArH),7.47 (d,2H,J = 7.8 Hz,ArH),7.73 (d,2H,J = 7.8 Hz,ArH),7.47 (d,2H,J = 7.8 Hz,ArH),13C NMR (75.5 MHz,DMSO-d6): δ 165.5,149.3,144.5,(C=N),134.5,133.3,132.1,131.2,130.7 129.4,129.2,128.4 (ArCs). Anal. Calcd. for C15H8Cl2N4S,C,51.89; H,2.32; N,16.14; S,9.23; found: C,51.86; H,2.35; N,16.15; S,9.22 EI-MS m/z: 345.0,347.0 (M+,100).
3-(4-Nitrophenyl-)-6-(4-nitrophenyl)-[1, 2, 4]triazolo[3,4- b][1, 3, 4]thiadiazole (5j): Reddish brown solid Rf = 0.26 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1 ): 2921 (Ar C-H str),2851 (methyl C-H str),1602 (N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSOd6): d 7.36 (d,1H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar-H); 7.36 (d,1H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar-H); 13C NMR (75.5 MHz,DMSO-d6): δ 166.3,152.0,146.5 (C=N),145.8,136.9,133.6,130.3,129.8,128.6,122.5,121.5 (ArCs). Anal. Calcd. for C1sH8N6O4S: C,48.91; H,2.19; N,22.82; S,8.71; found: C,48.89; H,2.21; N,22.80; S,8.73. EI-MS m/z: 368 (M+,100).
6-(4-Nitrophenyl)-3-p-tolyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5k): Yellow solid; Rf = 0.37 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1): 2921 (Ar C-H str),2851 (methyl C-H str),1602 (N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 7.36 (d,1H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar-H); 7.43 (d,2H,J = 7.5 Hz,Ar-H),8.13 (d,1H,J = 7.5 Hz,Ar-H),2.43 (s,3H,ArCH3). 13C NMR (75.5 MHz,DMSO-d6): δ 166.0,143.9,134.0 (C=N),133.9,132.7,131.1,130.5,129.7,129.4,128.3,127.7 (ArCs),22.= (CH3),Anal. Calcd. for C16H11N5O2S: C,56.96; H,3.29; N,20.76,S,9.50; found: C,56.94; H,3.31; N,20.74; S,9.52. EI-MS m/z: 337.0,339.0 (M+,100).
3-(4-Nitrophenyl-)-6-(3,4,5-trimethoxyphenyl)-[1, 2, 4]triazolo [3, 4-b][1, 3, 4]thiadiazole (5l): Yellow solid,Rf = 0.37 (petroleum ether:ethyl acetate = 7:3); IR (KBr,cm-1 ): 2921 (Ar C-H str),2851 (methyl C-H str),1602 (N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 7.36 (d,2H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar-H); 6.63 (s,2H,3,4,5-trimethoxybenzyl-H),3.61 (s,6H,3,5- di-CH3O),3.82 (s,3H,4-CH3O),13C NMR (75.5 MHz,DMSO-d6): d 167.3,151.0,143.5 (C=N),151.8,151.5,146.9,141.6,136.3,129.8,125.6,122.5,106.9 (ArCs). 56.7,56.4,54.2 (OCH3),Anal. Calcd. for C18H15N5O5S: C,52.30; H,3.66; N,16.94; S,7.76; found: C,52.28; H,3.68; N,16.92; S,7.78. EI-MS m/z: 413 (M+,100).
3-(4-Nitrophenyl-)-6-(4-pyridyl)-[1, 2, 4]triazolo[3, 4-b][1, 3, 4] thiadiazole (5m): Yellow solid; Rf = 0.3 (Petroleum ether: ethyl acetate = 7:3); IR (KBr,cm-1 ): 2921 (Ar,C-H str),2851 (methyl C- H str),1602 (N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 7.92,8.76 (m,4H,Py),7.24-7.28 (m,4H,J = 12 Hz; Ar-H). 13C NMR (75.5 MHz,DMSO-d6): δ 166.5 151.7,142.7,(C=N),133.2,132.5,129.6,128.9,127.5,126.3,120.4,(ArCs). Anal. Calcd. for C14H8N6O2S,C,51.85; H,2.49; N,25.91,S,9.89 found: C,51.83; H,2.51; N,25.90; S,9.90. EI-MS m/z: 324 (M+,100).
3-(4-Nitrophenyl-)-6-(2-chlorophenyl)-[1, 2, 4]triazolo[3, 4-b] [1, 3, 4]thiadiazole (5n): Light yellow solid,Rf = 0.31 (Petroleum ether: ethyl acetate = 7:3); IR (KBr,cm-1 ): 2921 (Ar C-H str),2851 (methyl C-H str),1602 (N=O str),1510 (C=N str),1492 (Ar,C=C str),1185 (Ar C-F str),990 (Ar-H bend); 1H NMR (300 MHz,DMSOd6): d 7.36 (d,1H,J = 8.1 Hz,Ar-H),8.36 (d,2H,J = 8.4 Hz,Ar-H),7.28-7.31 (m,4H,J = 9 Hz,Ar-H). 13C NMR (75.5 MHz,DMSO-d6): d 165.5,149.3,144.5,(C=N),142.4,138.3,137.3,136.6,133.8,132.8,131.8 130.4,129.2,122.4,(ArCs); Anal. Calcd. for C15H8ClN5O2S,C,50.36; H,2.25; N,19.57 S,8.96 found: C,50.34; H,2.27; N,19.57; S,8.96 EI-MS m/z: 357.0,359.0 (M+,100).
3-(4-Chlorophenyl)-6-p-tolyl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (5o): Yellow solid,Rf = 0.68 (Petroleum ether: ethyl acetate = 7:3) IR (KBr,cm-1): 3064 (Ar C-H str),2923 (methyl C-H str),1517 (C=N str),1466 (Ar,C=C str),971 (Ar-H bend); 1H NMR (300 MHz,DMSO-d6): δ 8.28 (d,2H,J = 7.5 Hz,Ar-H),8.10 (d,1H,J = 7.5 Hz,Ar-H),7.73 (d,2H,ArH,J = 7.8 Hz),7.47 (d,2H,J = 7.8 Hz,ArH),2.41 (s,3H,CH3),13C NMR (75 MHz,DMSO-d6): d 167.0,140.9,133.0 (C=N),132.9,131.7,130.1,130.0,129.8,129.6,128.5,127.3,(ArCs),21.5 (CH3); Anal. Calcd. for C16H11ClN4S: C,58.80; H,3.39; N,17.14; S,9.81 found: C,58.78; H,3.41; N,17.12; S,9.83. EI-MS m/z: 326 (M+,100).
3. Results and discussionThe synthesis of coupling partners 6-aryl-[1, 2, 4]triazolo[3,4- b][1, 3, 4]thiadiazoles (4a-o) was achieved according to the synthetic strategy outlined in Scheme 1. Formic acid (1) was treated with thiocarbohydrazide (2) using dry ethanol as a solvent to afford 4-amino-1,2,4-triazole-3-thiole (3),which was further reacted with suitably substituted aromatic acids in the presence of phosphoryl chloride to afford the targeted compounds (5a-o) (Scheme 2).
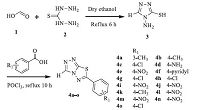
|
Download:
|
| Scheme 1.Synthesis of 6-aryl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazole (4a-o). | |
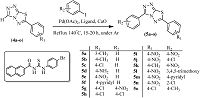
|
Download:
|
| Scheme 2.Coupling of 4a-o to aryl iodides leading to 3,6-diaryl-[1, 2, 4]triazolo[3, 4-b] [1, 3, 4] thiadiazoles (5a-o). | |
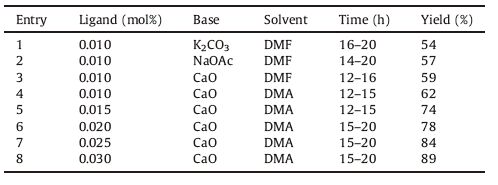
The phophine-free Pd-catalyzed cross-coupling of (4a-o) with suitably substituted aryl iodides was carried out in the presence of 1-(2-naphthoyl)-3-(4-bromophenyl)thiourea as a ligand to afford the targeted compounds 3,6-diaryl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4] thiadiazoles (5a-o) in 78%-89% yields. The reaction conditions for the synthesis of compound 5a were optimized originally by screening for the best solvent,base,refluxing time,mol percent of thiourea ligand constant (entries 4-8) and then the optimum mol percent of ligand was established (Table 1).
|
|
Table 1 Optimization table for the synthesis of 5a. |
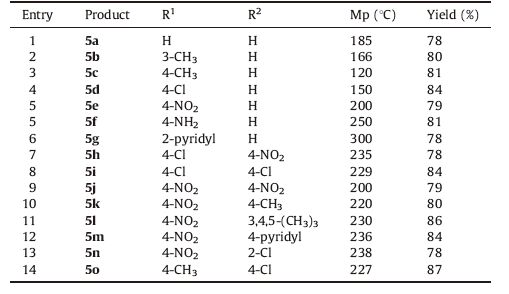
Once the best conditions were determined the coupling was carried out between iodoanilines with 4a-o in N,N-dimethylacetamide (DMA) using CaO as a base and 0.03 mol% of the catalyst to afford the desired products (5a-o) in good to excellent yields (Table 2).
|
|
Table 2 3,6-Diaryl-[1, 2, 4]triazolo[3, 4-b][1, 3, 4]thiadiazoles 5a-o produced via Scheme 1. |
1H NMR spectroscopy,ESI-MS spectra and elemental analysis data of the targeted compounds fully accorded with their assigned structures. The FTIR spectra of all compounds showed strong absorptions around 3016,2959 cm-1 for C-H stretchings,in addition to the peaks at 3115-3100 cm-1 and 1618-1605 cm-1 assignable to C=C and C=N functions,respectively. In the 1H NMRspectral data for all the compounds,the characteristic thiazolyl proton disappeared and only aromatic protons were found. The 13C NMR spectral data show deshielded signals for C=N of thiazole and triazole in the range of δ 164.6-162.0 and δ 159.9-158.3,respectively,due to the neighboring sulfur and nitrogen atoms. The purity and homogeneity of all compounds were confirmed by their sharp melting points and analytical TLC.
4. ConclusionThe present study offers an efficient and convenient phosphinefree C-H activated arylation of the triazol-H of triazolo-thiadiazoles using thiourea/Pd catalysis. The yields are satisfactory and the thiourea ligand used is thermally stable,insensitive to air and moisture,and tunable by modifying the substituents on the aryl ring.
| [1] | R.F. Heck, J.P. Nolley Jr., Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides, J. Org. Chem. 37(1972) 2320-2322. |
| [2] | T. Mizoroki, K. Mori, A. Ozaki, Arylation of olefin with aryl iodide catalyzed by palladium, Bull. Chem. Soc. Jpn. 44(1971) 581-587. |
| [3] | C. Shen, P. Zhang, Q. Sun, et al., Recent advances in C-S bond formation via C-H bond functionalization and decarboxylation, Chem. Soc. Rev. 44(2015) 291-314. |
| [4] | X. Cai, B. Xie, Recent advances in nickel-catalyzed C-H bond functionalized reactions, ARKIVOC(i)(2015) 184-211. |
| [5] | Z.Q. Lin, W.Z. Wang, S.B. Yan, Palladium-catalyzed enantioselective CH arylation for the synthesis of P-stereogenic compounds, Angew. Chem. Int. Ed. 54(2015) 6265-6269. |
| [6] | M. Moselage, N. Sauermann, S.C. Richter, et al., C-H alkenylations with alkenyl acetates, phosphates, carbonates, and carbamates by cobalt catalysis at 23℃, Angew. Chem. Int. Ed. 54(2015) 6352-6635. |
| [7] | S. Protti, M. Fagnoni, D. Ravelli, Photocatalytic CH activation by hydrogen-atom transfer in synthesis, ChemCatChem 7(2015) 1516-1523. |
| [8] | X. Sun, Y. Sun, Y. Rao, A gram-scale synthesis of multi-substituted arenes via palladium catalyzed C-H halogenation, Chin. Chem. Lett. 25(2014) 667-669. |
| [9] | L.Y. Liu, Y.Z. Yan, Y.J. Bao, Z.Y. Wang, Efficient synthesis of 2-arylquinazolines via copper-catalyzed dual oxidative benzylic C-H aminations of methylarenes, Chin. Chem. Lett.(2015), http://dx.doi.org/10.1016/j.cclet.2015.07.008. |
| [10] | D. Chen, H. Mo, D. Chen, J. Yang, Direct C-H amination for indole synthesis from NTs-2-styrylaniline derivatives catalyzed by copper salt, Chin. Chem. Lett. 26(2015) 969-972. |
| [11] | D. Yang, Y. Chen, N. Zhu, Sterically bulky thioureas as air-and moisture-stable ligands for Pd-catalyzed Heck reactions of aryl halides, Org. Lett. 6(2004) 1577-1580. |
| [12] | D. Mingji, B. Liang, C. Wang, et al., A novel thiourea ligand applied in the Pdcatalyzed Heck, Suzuki and Suzuki carbonylative reactions, Adv. Synth. Catal. 346(2004) 1669-1673. |
| [13] | S. Keesara, S. Parvathaneni, M.R. Mandapati, N,N'-Mono substituted acyclic thioureas:efficient ligands for the palladium catalyzed Heck reaction of deactivated aryl bromides, Tetrahedron Lett. 55(2014) 6769-6772. |
| [14] | S.M.I. Badr, R.M. Barwa, Synthesis of some new[1,2,4] triazolo[3,4-b][1,3,4] thiadiazines and[1,2,4] triazolo[3,4-b][1,3,4] thiadiazoles starting from 5-nitro-2-furoic acid and evaluation of their antimicrobial activity, Bioorg. Med. Chem. 19(2011) 4506-4512. |
| [15] | R.D. Hunashal, D. Satyanarayana, One pot synthesis of 3-(substituted phenoxymethyl)-6-phenyl/substituted phenoxymethyl-1,2,4-triazolo[3,4-b][1,3,4] thiadiazole derivatives as antimicrobial agents, Int. J. Pharm. Biol. Sci. 3(2012) 183-192. |
| [16] | A. Saeed, S. Ashraf, J.M. White, et al., Synthesis, X-ray crystal structure, thermal behavior and spectroscopic analysis of 1-(1-naphthoyl)-3-(halo-phenyl)-thioureas complemented with quantum chemical calculations, Spectrochim. Acta 143(2015) 59-66. |
 2016, Vol.27
2016, Vol.27