b Department of Anal-colorectal Surgery, the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310006, China;
c Leibniz-Institut für Katalyse e. V. an der Universit ät Rostock, 18059 Rostock, Germany
Quinazolinones represent a high value class of compounds in organic chemistry,which has a wide range of biological and pharmacological activities [1],including anti-bacterial [2],anticancer [3],anti-inflammatory [4],anti-microbial [5],anti-tubercular [6],anti-ulcer [7],and so on [8]. Additionally,they are important intermediates in natural products preparation [9] and applied as structural scaffold in drug discovery.
Owing to their diverse bioactivity and privileged sub-structure for drug design,the synthetic methodologies development becomes attractive and many synthetic routes have been developed [10a]. For example,copper-catalyzed Ullmann-type coupling reactions [10b],redox condensation between o-substituted nitrobenzenes with amines in the presence of iron and cobalt as catalysts [11],one-pot synthesis of quinazolinones from benzyl alcohols by iridium or ruthenium catalysts [12]. In these reactions,metal-salts turned out to be indispensable,and many metal wastes were generated. Besides,condensation between o-aminobenzamides and aldehydes with additional oxidants is also a typical method to prepare quinazolinones [13]. Recently,a procedure on autoxidation of benzyl amines and applied in heterocycles synthesis was developed as well [14a]. Five examples of quinazolinones were prepared in moderate yields under 150 ℃ with 40% AcOH as the additive. Meanwhile,we reported a procedure for the transformation of benzyl amines to the corresponding imines [15]. Various imines were produced in good to excellent yields under metal-free conditions and with H2O2 as the green oxidant. From mechanistic point of view,the oxidation of benzyl amines to the corresponding benzaldehydes should be the first step and then followed by condensation to give the imines. Hence,we believe a procedure for quinazolinones preparation with o-aminobenzamides and benzylamines as the substrates without metal catalysts and using H2O2 as the green oxidant should be highly realizable. Under this context,we developed this interesting metal-free and additive-free procedure for the synthesis of quinazolinones from o-aminobenzamides and benzyl amines.
2. ExperimentalA 15 mL tube was added 2-aminobenzamide (1 mmol),benzyl amine (1.5 mmol),and a stir bar. Then H2O2 (30 wt% in H2O,5 equiv.) was added by a syringe at room temperature under open air. The tube was closed and kept at 120 ℃ for 20 h. The conversion and yield were determined by GC and GC-MS using hexadecane (0.1 mmol) as the internal standard.
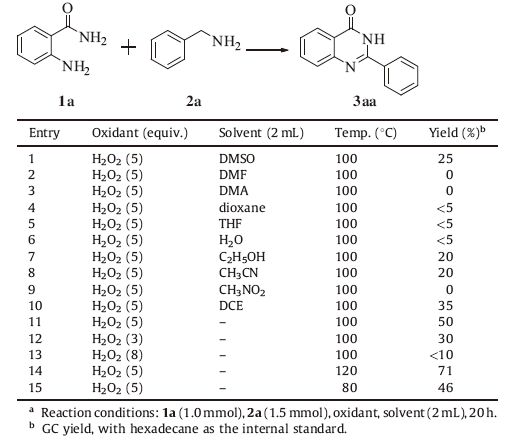
3. Results and discussionAt the beginning,substrates 1a and 2a were chosen as the starting materials. The reaction was conducted with 5 equiv. of H2O2 (30 wt% in H2O) at 100 ℃ in DMSO,fortunately,25% yield of the target product 3aa was obtained (Table 1,entry 1). Encouraged by this result,we continued with the studies of different solvents which did not improve the yield significantly (Table 1,entries 2- 10). However,when we tried the reaction without solvent,the yield raised to 50% (Table 1,entry 11). Furthermore,the amount of H2O2 was examined,less and more H2O2 all decreased the yield (Table 1,entries 12 and 13). Notably,reaction temperature was found hold great influence for this transformation. 80 ℃ resulted in 46% yield (Table 1,entry 15),while 120 ℃ resulted in 71% yield (Table 1,entry 14). No better yield can be obtained under higher reaction temperature.
|
|
Table 1 Screening of the reaction conditions.a |
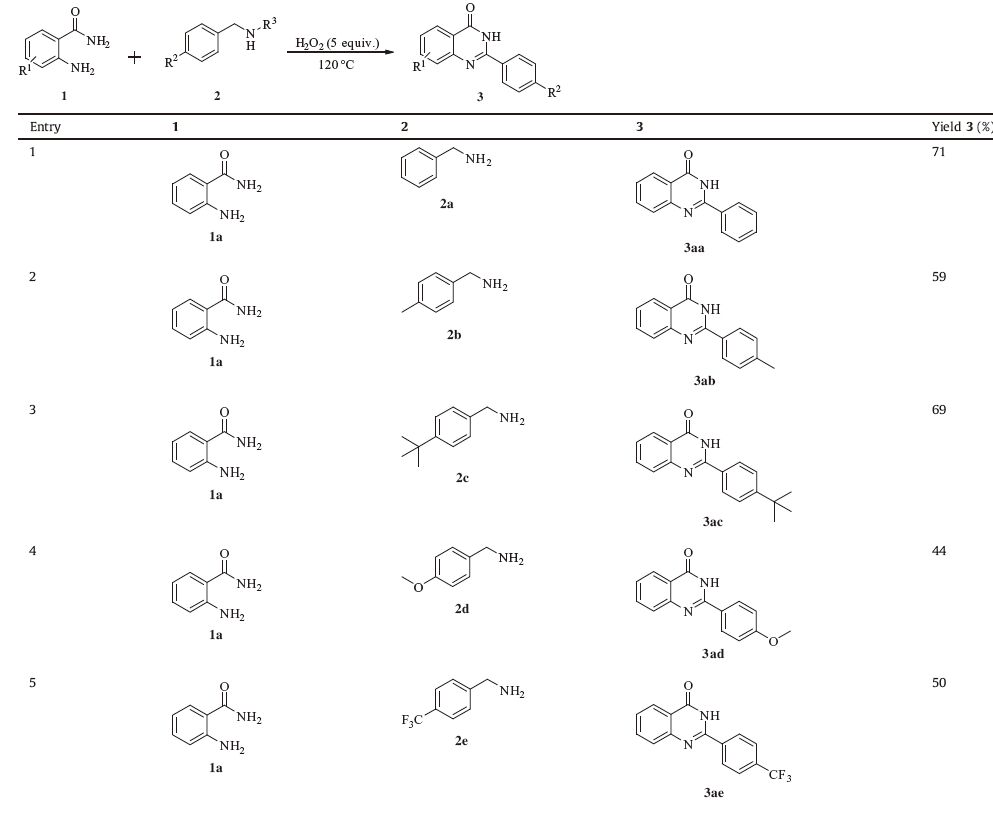
Inspired by this result,we next investigated the substrates scope. First,o-aminobenzamide and a variety of para-substituted benzyl amines were studied (Table 2,entries 1-10). The substrates with electron-donating group,such as methyl,tert-butyl group smoothly afford the quinazolinones in 59% and 69% yield (Table 2,entries 2 and 3). However,as an electron-rich group,paramethoxybenzylamine decreased the yield of the desired product to 44% (Table 2,entry 4). A moderate product yield was generated when the substrate decorated with electron-deficient group,such as trifluoromethyl moiety (Table 2,entry 5). Moreover,benzyl amines bearing fluoro,chloro,and bromo provided the desired products with slightly lower yields (Table 2,entries 6-8). It is noteworthy that heteroaromatic substrate can also be tolerated under the standard condition,resulted the corresponding product in 65% yield (Table 2,entry 9). In addition,N-substituted benzylamine was also examined,affording the product 3aa in 45% yield (Table 2,entry 10). However,aliphatic amines such as butylamine and 2-phenylethanamine failed in our system. Additionally,no desired product was observed with N,N-dimethylbenzyl amine.
|
|
Table 2 Synthesis of quinazolinones: substrates testing.a |
Next,a series of o-aminobenzamides were then subjected to the optimized reaction conditions,and the results were summarized (Table 2,entries 11-14). We were delighted to find that all the oaminobenzamides examined worked well and succeeded to give the target products in moderate yields.
Regarding the reaction pathway,a possible reaction mechanism has been proposed in Scheme 1. First,benzyl amine 1 was oxidized to benzaldehyde in the presence of H2O2. Then,the condensation of the in situ formed aldehyde with o-aminobenzamide 2 occurred. The condensed intermediate can provide the final quinazolinone 3 after further oxidation step.
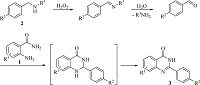
|
Download:
|
| Scheme 1. Proposed reaction mechanism | |
In conclusion,we have developed an environmental friendly strategy for the quinazolinones preparation. With o-aminobenzamides and benzyl amines as the substrates and H2O2 as the green oxidants under metal-free and solvent-free conditions,a variety of quinazolinones were generated in moderate to good yields.
AcknowledgmentsThe authors thank the financial supports from NSFC (No. 21472174),Education Department of Zhejiang Province (No. Y201432060) and Zhejiang Sci-Tech University (Nos. 1206838-Y and 14062015-Y). X.-F. Wu appreciates the general support from Matthias Beller in LIKAT.
| [1] | (a) S.B. Mhaske, N.P. Argade, The chemistry of recently isolated naturally occurring quinazolinone alkaloids, Tetrahedron 62(2006) 9787-9826;(b) D.A. Horton, G.T. Bourne, M.L. Smythe, The combinatorial synthesis of bicyclic privileged structures or privileged substructures, Chem. Rev. 103(2003) 893-930. |
| [2] | A.K. Nanda, S. Ganguli, R. Chakraborty, antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3h)-ones:synthesis and preliminary QSAR Studies, Molecules 12(2007) 2413-2426. |
| [3] | (a) P.M. Chandrika, T. Yakaiah, A.R.R. Rao, et al., Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity(cytotoxic) against U937 leukemia cell lines, Eur. J. Med. Chem. 43(2008) 846-852;(b) S.L. Cao, Y.P. Feng, Y.Y. Jiang, et al., Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains, Bioorg. Med. Chem. Lett. 15(2005) 1915-1917;(c) M. Dupuy, F. Pinguet, O. Chavignon, et al., Synthesis and in vitro cytotoxic evaluation of new derivatives of pyrido[1,2-a]benzimidazolic ring system:the pyrido[1',2':1,2] imidazo[4,5-h]quinazolines, Chem. Pharm. Bull. 49(2001) 1061-1065;(d) Y. Takase, T. Saeki, N. Watanabe, et al., Cyclic GMP phosphodiesterase inhibitors. 2. Requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity, J. Med. Chem. 37(1994) 2106-2111. |
| [4] | O. Kenichi, Y. Yoshihisa, O. Toyonari, I. Toru, I. Yoshio, Studies on 4(1h)-quinazolinones. 5. Synthesis and antiinflammatory activity of 4(1h)-quinazolinone derivatives, J. Med. Chem. 28(1985) 568-576. |
| [5] | B.S. Kuarm, Y.T. Reddy, J.V. Madhav, P.A. Crooks, B. Rajitha, 3-[Benzimidazo]-and 3-[benzothiadiazoleimidazo-(1,2-c)quinazolin-5-yl]-2H-chromene-2-ones as potent antimicrobial agents, Bioorg. Med. Chem. Lett. 21(2011) 524-527. |
| [6] | K. Waisser, J. Gregor, H. Dostal, et al., Influence of the replacement of the oxo function with the thioxo group on the antimycobacterial activity of 3-aryl-6,8-dichloro-2h-1,3-benzoxazine-2,4(3h)-diones and 3-arylquinazoline-2,4(1h, 3h)-diones, Farmaco 56(2001) 803-807. |
| [7] | K. Tereshima, H. Shimamura, A. Kawase, et al., Studies on antiulcer agents. IV. Antiulcer effects of 2-benzylthio-5,6,7,8-tetrahydro-4(3h)-quinazolinones and related compounds, Chem. Pharm. Bull. 43(1995) 2021-2023. |
| [8] | (a) H. Kikuchi, K. Yamamoto, S. Horoiwa, et al., Exploration of a new type of antimalarial compounds based on febrifugine, J. Med. Chem. 49(2006) 4698-4706;(b) N. Malecki, P. Carato, G. Rigo, et al., Synthesis of condensed quinolines and quinazolines as DNA ligands, Bioorg. Med. Chem. 12(2004) 641-647;(c) K. Matsuno, J. Ushiki, T. Seishi, et al., Potent and selective inhibitors of plateletderived growth factor receptor phosphorylation. 3. Replacement of quinazoline moiety and improvement of metabolic polymorphism of 4-[4-(N-substituted(thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives, J. Med. Chem. 46(2003) 4910-4925. |
| [9] | J.F. Liu, Rapid syntheses of biologically active quinazolinone natural products using microwave technology, Curr. Org. Syn. 4(2007) 223-237. |
| [10] | (a) L. He, H. Li, J. Chen, X.F. Wu, Recent advances in 4(3h)-quinazolinone syntheses, RSC Adv. 4(2014) 12065-12077;(b) W. Xu, Y. Jin, H. Liu, Y. Jiang, H. Fu, Copper-catalyzed domino synthesis of quinazolinones via ullmann-type coupling and aerobic oxidative C-H amidation, Org. Lett. 13(2011) 1274-1277;(c) W. Xu, H. Fu, Amino acids as the nitrogen-containing motifs in coppercatalyzed domino synthesis of N-heterocycles, J. Org. Chem. 76(2011) 3846-3852;(d) B.Q. Hu, L.X. Wang, J.F. Xiang, L. Yang, Y.L. Tang, Cu(Ⅱ)-catalyzed domino reaction of 2-halobenzamide and arylmethanamine to construct 2-aryl quinazolinone, Chin. Chem. Lett. 26(2015) 369-372;(e) M. Wang, T.T. Zhang, Z.G. Song, Eco-friendly synthesis of 2-substituted-2,3-dihydro-4(1h)-quinazolinones in water, Chin. Chem. Lett. 22(2011) 427-430;(f) M. Wang, Z.G. Song, T.T. Zhang, Strontium chloride-catalyzed one-pot synthesis of 4(3H)-quinazolinones under solvent-free conditions, Chin. Chem. Lett. 21(2010) 1167-1170;(g) C. Xie, H.X. Li, M.G. Liu, M.W. Ding, Efficient synthesis of 4(3h)-quinazolinones using a soluble polymeric support, Chin. Chem. Lett. 19(2008) 505-508. |
| [11] | T.B. Nguyen, J.L. Bescont, L. Ermolenko, A. Al-Mourabit, Cobalt-and iron-catalyzed redox condensation of o-substituted nitrobenzenes with alkylamines:a step-and redox-economical synthesis of diazaheterocycles, Org. Lett. 15(2013) 6218-6221. |
| [12] | (a) A.J.A. Watson, A.C. Maxwell, J.M. Williams, Ruthenium-catalysed oxidative synthesis of heterocycles from alcohols, Org. Biomol. Chem. 10(2012) 240-243;(b) J. Zhou, J. Fang, One-pot synthesis of quinazolinones via iridium-catalyzed hydrogen transfers, J. Org. Chem. 76(2011) 7730-7736. |
| [13] | (a) T. Hisano, M. Ichikawa, A. Nakagawa, M. Tsuji, Studies on organosulfur compounds. Ⅻ. Syntheses and pharmacological activities of 2-heterocyclic substituted 4(3h)-quinazolinones, Chem. Pharm. Bull. 23(1975) 1910-1916;(b) R.J. Abdel-Jalil, H.M. Aldoqum, M.T. Ayoub, W. Voelter, Synthesis and antitumor activity of 2-aryl-7-fluoro-6-(4-methyl-1-piperazinyl)-4(3h)-quinazolinones, Heterocycles 65(2005) 2061-2070;(c) M. Bakavoli, A. Shiri, Z. Ebrahimpour, M. Rahimizadeh, Clean heterocyclic synthesis in water:I2/KI catalyzed one-pot synthesis of quinazolin-4(3h)-ones, Chin. Chem. Lett. 19(2008) 1403-1406;(d) C. Balakumar, P. Lamba, D.P. Kishore, et al., Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines, Eur. J. Med. Chem. 45(2010) 4904-4913. |
| [14] | (a) T.B. Nguyen, L. Ermolenko, A. Al-Mourabit, Selective autoxidation of benzylamines:application to the synthesis of some nitrogen heterocycles, Green Chem. 15(2013) 2713-2717;(b) D. Mao, J. Tang, W. Wang, et al., A Sc(OTf)3-catalyzed cascade reaction of oaminoacetophenone with methanamine:construction of dibenzo[b,h][1,6]-naphthyridine derivatives, Org. Biomol. Chem. 13(2015) 2122-2128;(c) D. Mao, J. Tang, W. Wang, et al., Scandium pentafluorobenzoate-catalyzed unexpected cascade reaction of 2-aminobenzaldehydes with primary amines:a process for the preparation of ring-fused aminals, J. Org. Chem. 78(2013) 12848-12854. |
| [15] | X.F. Wu, A. Petrosyan, T.V. Ghochikyan, A.S. Saghyan, P. Langer, Metal-free oxidation of benzyl amines to imines, Tetrahedron Lett. 54(2013) 3158-3159. |
 2016, Vol.27
2016, Vol.27