The development of new synthetic methods leading to hybrid structures,which consist of different biologically active moieties in a single molecule has attracted much attention. The heterocyclic pharmacophores are selected on the basis of their known bio profiles,so that the successive hybrid molecules may exhibit synergistic or additive pharmacological activities [1, 2]. Imidazoles are quite attractive due to their biological activities [3, 4, 5]. Fused imidazoles are described to have antibacterial,current antiviral therapy for chronic hepatitis C,antifungal and perspectives of drug design that targets RNA [6]. Moreover,the structural features of these compounds are found in nature and are incorporated as key structural fragments in many biological and chemical systems [7]. Broad range of therapeutic drugs has also been developed like Zolmidine,Zolpidem,Alpidems and Kifunersine [8]. Temozolomide is used for the treatment of refractory brain tumors [9, 10, 11]. Isoxazole derivatives are reported with diverse structural features and versatile biological properties such as antitumor [12],CNSactive [13],analgesic [14],antimicrobial [15],relaxant [16],for the treatment of hypercholsteremia and hyperlipidemia [17],as synthetic muscle intermediates [18],and as chemotherapeutic agents [19]. Based on the bio activities of imidazole and isoxazole ring systems,we proposed to construct a system that combine these bio-labile rings together in a single molecular frame work. The synthesis of imidazo[2,1-b]thiazoles are reported earlier and these compounds showed promising biological activity [20, 21]. Based on these reports,and as a sequel to our work on the synthesis of fused isoxazoles [22],we herein,wish to report the synthesis of novel imidazo[1,2-b]isoxazoles and their Mannich bases as potential biodynamic agents.
2. ExperimentalAll the melting points were determined on a Cintex melting point apparatus and are uncorrected. Analytical TLC was performed on Merck precoated 60 F254 silica gel plates. Visualization was done by exposing to iodine vapour. IR spectra (KBr pellet) were recorded on a Perkin-Elmer BX series FT-IR spectrometer. 1H NMR spectra were recorded on a Varian Gemini 300 MHz spectrometer. 13C NMR spectra were recorded on a Bruker 75 MHz spectrometer. Chemical shift values are given in δ ppm with tetramethyl silane as an internal standard. Mass spectral measurements were carried out by EI method on a Jeol JMC-300 spectrometer at 70 eV. Elemental analyses were performed on a Carlo Erba 106 and Perkin-Elmer model 240 analyzers.
2.1. General procedure for the synthesis of 6-methyl-3-aryl imidazolo[1, 2, b]isoxazoles 3a-dA solution of 3-amino-5-methylisoxazole 1 (0.01 mol),and vbromoacetophenone 2 (0.01 mol) in dry ethanol (20 mL) was refluxed for 8 h. After completion of the reaction (monitored by TLC),the reaction mixture was poured into 25 mL of saturated NaHCO3 solution with stirring. This mixture was extracted with chloroform (3 × 20 mL),and the combined organic layers were distilled under reduced pressure. The resulting solid was purified by recrystallization from ethyl acetate.
2.2. General procedure for the synthesis of 6-methyl-3-aryl-2- (morpholino/pyrrolidin-1-yl/piperidin-1-yl-methylimidazo[1, 2, -b] isoxazoles 4a-d,5a-d and 6a-dA solution of 6-methyl-3-aryl imidazo[1,2-b]isoxazoles 3 (0.01 mol) in acetic acid (20 mL) was added dropwise to a stirred solution of morpholine (0.01 mol)/pyrrolidine (0.01 mol)/piperidine (0.01 mol),37% formaline (0.01 mol) and acetic acid (15 mL) in methanol (20 mL). Termination of the reaction was monitored by TLC. The reaction mixture was then poured into 20 mL of 10% Na2CO3 solution with stirring. This mixture was then extracted with chloroform (3 × 20 mL),and the combined organic layers were distilled under reduced pressure. The resulting solid was purified by recrystallization from ethanol.
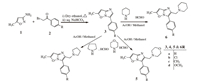
3. Results and discussionThe synthesis of title compounds viz.,6-methyl-3-aryl imidazo[ 1,2-b]isoxazoles 3 were achieved by the reaction of 3-amino- 5-methylisoxazole 1 with substituted phenacyl bromides 2 in dry ethanol followed by treatment with aqueous NaHCO3.
Mannich reaction of 6-methyl-3-aryl imidazo[1,2-b] isoxazoles 3 with 2°-amines like morpholine/pyrrolidine/piperidine,and formaline resulted in the formation of Mannich bases viz 6- methyl-3-aryl-2-(morpholino/pyrrolidin-1-yl/piperidin-1-yl)-methylimidazo[ 1,2,-b]isoxazoles 4,5 and 6 in excellent yields (Scheme 1).
|
Download:
|
| Scheme. 1.Synthesis of 6-methyl-3-aryl imidazolo[1,2-b]isoxazoles and their Mannich bases. | |
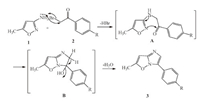
The plausible mechanism for the formation of 6-methyl-3-aryl imidazole[1,2-b]isoxazoles 3 is depicted in the Scheme 2. The amino group of isoxazole makes a nucleophilic attack on bromine bearing carbon there by displaces HBr to give intermediate A. The C55N group of isoxazole activated by NH group attacks carbonyl group and undergoes intramolecular cyclization to give compound B,which then undergoes spontaneous dehydration by the action of NaHCO3 to afford the title compounds. The structures of the products 3-6 have been elucidated on the basis of spectral (IR,1H NMR,13C NMR and MS) and micro analytical data.
|
Download:
|
| Scheme. 2.Plausible mechanism for the formation of 6-methyl-3-aryl imidazo[1,2- b]isoxazoles. | |
The present study offers a facile and convenient method for the synthesis of imidazo[1,2-b]isoxazoles and their Mannich bases using inexpensive and commercially available materials. The title compounds may act as drug candidates based on the pharmacological activity of isoxazole and imidazole moieties.
AcknowledgmentsThe authors are thankful to the Head,Department of Chemistry, Kakatiya University,Warangal for facilities and to the Director, Indian Institute of Chemical Technology,Hyderabad for recording 1H NMR,13C NMR and Mass Spectra.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.07.024.
| [1] | E.M. Guantai, K. Ncokazi, T.J. Egan, et al., Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds, Bioorg. Med. Chem. 18 (2010) 8243-8256. |
| [2] | C.A. Fraga, Drug hybridization strategies: before or after lead identification? Expert Opin. Drug Discov. 4 (2009) 605-609. |
| [3] | F. Bellina, S. Cauteruccio, S. Montib, R. Rossi, Novel imidazole-based combretastatin A-4 analogues: Evaluation of their in vitro antitumor activity and molecular modelling study of their binding to the colchicines site of tubulin, Bioorg. Med. Chem. Lett. 16 (2006) 5757-5762. |
| [4] | J.Z. Vlahakis, M. Humb, M.N. Rahman, et al., Synthesis and evaluation of imidazole- dioxolane compounds as selective heme oxygenase inhibitors: Effect of substituents at the 4-position of the dioxolane ring, Bioorg. Med. Chem. 17 (2009) 2461-2475. |
| [5] | K. Bhandari, N. Srinivas, V.K. Marrapu, A. Verma, S. Srivastava, S. Gupta, Synthesis of substituted aryloxy alkyl and aryloxy aryl alkyl imidazoles as antileishmanial agents, Bioorg. Med. Chem. Lett. 20 (2010) 291-293. |
| [6] | K. Hanazaki, P. Gupta, S.K. Singh, et al., Current antiviral therapy for chronic hepatitis C including liver transplantation and hepatic resection, Curr. Med. Chem. 2 (2003) 103-111. |
| [7] | K. Bleicher, F. Gerber, Y. Wuthrich, A. Alanine, A. Capretta, Parallel synthesis of substituted imidazoles from 1,2-aminoalcohols, Tetrahedron Lett. 43 (2002) 7687-7690. |
| [8] | (a) G. Trapani, M. Franco, A. Latrofa, et al., Derivatives of imidazole. III. Synthesis and pharmacological activities of nitriles, amides, and carboxylic acid derivatives of imidazo[[1,2-a] pyridines, J. Med. Chem. 42 (1999) 3934-3937; (b) G.J. George, D.P. Vercauteren, G.H. Evard, et al., Characterization of the physicchemical properties of the imidazopyridine derivative Alpidem, Comparison with Zolpidem, Eur. J. Med. Chem. 28 (1993) 323-325. |
| [9] | S.M. Reilly, E.S. Newlands, M.G. Glaser, et al., Temozolomide: A new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumors, Eur. J. Cancer 29A (1993) 940-942. |
| [10] | H.S. Friedman, T. Kerby, H. Calvert, Temozolomide and treatment of malignant glioma, Clin. Cancer Res. 6 (2000) 2585-2597. |
| [11] | T. Batchelor, Primary nervous-system lymphoma, Lancet 355 (2000) 354-365. |
| [12] | J. Getal, Synthesis of 3-[1,3-thazol-2-yl-] as potential antitumor agents, Antibiotics 28 (1975) 91-94. |
| [13] | C.H. Eugster, Chemie der wirkstoffe aus dem fliegenpilz (amanita muscaria), Prog. Chem. Org. Nat. Prod. 27 (1969) 261. |
| [14] | H. Kano, I. Adachi, R. Kido, K. Hirose VIII., Synthesis and pharmacological properties of 5-aminoalkyl- and 3-aminoalkylisoxazoles and related derivatives, J. Med. Chem. 10 (1967) 411-418. |
| [15] | P.B. Reddy, S.M. Reddy, E. Rajanarendar, A.K. Murthy, Antifungal activity of isoxazolyl thiazolidin-4-ones, Indian Phytopathol. 37 (1984) 369-398. |
| [16] | M.T. Chemicals, K.K. Mitsui, Seiyaku kogyoo, vol. 6, Toyama Chemical Co. Ltd., 1994p. 146. |
| [17] | E.T. Marquis, J.R. Sanderson, US Patent 528335 (1994), Chem. Abstr. 120 (1994) 217649. |
| [18] | Streling Drug. Inc., US Patent (1988) 4755595, Chem. Abstr. 110 (1989) 730. |
| [19] | A. Sadanadam, M.V. Rajam, K. Subash, E. Rajanarendar, Production of chromosomal breaks by isoxazolyl thiazolidin-one in allium sativu, Indian Bot. Rep. 3 (1984) 38-42. |
| [20] | F. Palagiano, L. Arenare, E. Luraschi, et al., Research on heterocyclic compounds XXXIV. Synthesis and SAR study of some imidazo[2,1-b] thiazole carboxylic and acetic acids with anti-inflammatory and analgesic activity, Eur. J. Med. Chem. 30 (1995) 901-909. |
| [21] | S. Kamila, K. Mendoza, E.R. Biehl, Microwave-assisted Hantzsch thiazole synthesis of N-phenyl-4-16-phenylimidazo[2, 1-b] thiazole-5-yl)-thiazol-2-amines from the reaction of 2-chloro-1-(6-phenylimidazo[2,1-b]thiazole-5-yl)ethenones and thioureas, Tetrahedron Lett. 53 (2012) 4921-4924. |
| [22] | (a) E. Rajanarendar, S. Ramakrishna, K. Ramamurthy, Synthesis of novel isoxazolyl bis-thiazolo[3,2-a]pyrimidines, Chin. Chem. Lett. 23 (2012) 899-902; (b) E. Rajanarendar, S. Ramakrishna, K. Govardhan Reddy, D. Nagaraju, Y.N. Reddy, A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl- 2,3-dihydrospiro [benzo[f]isoindole-1,3'-indoline]-2',4,9-triones, Bioorg. Med. Chem. Lett. 23 (2013) 3954-3958; (c) E. Rajanarendar, K. Govardhan Reddy, S. Rama Krishna, et al., Synthesis and in vitro and in vivo anticancer activity of novel 3-methyl-5H-isoxazolo[ 5'4;:5,6]pyrido[2,3-b]indoles, Bioorg. Med. Chem. Lett. 22 (2012) 6677-6680; (d) E. Rajanarendar, G. Mohan, E. Kalyan Rao, M. Srinivas, Palladium-catalyzed Suzuki-Miyaura cross-coupling reaction of organo boronic acids with N-protected 4-iodophenyl alanine linked isoxazoles, Chin. Chem. Lett. 1 (2009) 1-4; (e) E. Rajanarendar, A. Siva Rami Reddy, S. Raju, S. Firoz Pasha, K. Govardhan Reddy, A fast and highly efficient protocol for reductive amination of aromatic aldehydes using NaBH4 and isoxazole amines in an ionic liquid medium, Chin. J. Chem. 29 (2011) 769-772. |
 2015, Vol.26
2015, Vol.26 


