C-N bond construction has been one of the most important research topic in organic synthesis,accompanied by the development of Ullmann reaction [1, 2] and Buchwald-Hartwig amination [3, 4]. However,their high cost and environmental toxicity,such as stoichiometric amounts of halogen salt as by-product,prevent the large-scale syntheses in industrial applications. Recently,the direct C-H bond functionalization has been more facility,straightforward and environment friendly protocols to construct carbon-carbon [5] and carbon-heteroatom bonds [6, 7, 8]. However,most of transitionmetal- catalyzed C-H amination reactions require stoichiometric external oxidants and the harsh reaction conditions. Organic azides,which is an environmental amino source and also as an internal oxidant viaN-N2 bondcleavage,would be key to develop an efficient C-H amination and the sole byproduct is molecular nitrogen (N2) [9, 10]. Recently,Chang reported elegantworks on azides as nitrogen source for transition-metal-catalyzed direct C-H amidations [11- 21]. Miura [22],Daugulis [23] and Yu [24, 25, 26, 27, 28, 29, 30, 31] have independently achieved direct ortho-C-H amidation of benzoic acids. Along with our continuing efforts to explore novel C-N bond formations [7],we herein independently reported an iridium-catalyzed carboxylic acid-directedC-Haminationswith sulfonyl azides as aminosources,which afforded sulfonamide and anthranilic acid derivatives [32]. Notably,the products obtained from this protocol as an important structural units widely exist in pharmaceuticalmolecules and natural products,such as the potent inhibitor of methionine aminopeptidase-2 (MetAP-2) (Fig. 1,left),and sulfonamide derivatives with efficiently anti-inflammatory and aldose reductase inhibitory activities (Fig. 1,right) [33, 34].
|
Download:
|
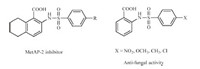
| Fig. 1.Examples illustrating the importance of 2-sulfonylamido benzoic acids. | |
To a screw capped vial with a spinvane triangular-shaped Teflon stir bar were added benzoic acids (1,0.20 mmol),azide (2,0.20 mmol),[IrCp*Cl2]2 (3.2 mg,4 mol%),AgNTf2 (6.2 mg,8 mol%),Li2CO3 (2.2 mg,15 mol%),AcOH(1.8 mg,15 mol%) and 1,2-dichloroethane (2.0 mL) under N2 atmosphere. The reaction mixture was stirredin apre-heatedoil bath at the indicatingtemperature for12 h. Then,thereactionmixturewas cooledtoroomtemperature incaseof heating,filtered througha padof celite and thenwashedwithCH2Cl2 (5 mL×3). The solventswereremovedin vacuo,andthe residuewas purified by column chromatography on silica gel (PE/EtOAc/ HCOOH = 30:1:0.1-25:5:0.1,v/v/v) to give the target product 3.
3. Results and discussionAt the outset ofour studies,themodel reactionof 2-fluorobenzoic acid (1a)withpara-methylphenylsulfonyl azide (2a) in the presence of [IrCp*Cl2]2 was chosen to screen the reaction parameters (Table 1). The reaction did not proceed in the absence of AgNTf2 or Ir(III) catalyst (entries 1-2). To our delight,LiOAc (15mol%),NaOAc (15 mol%) or LiOAc (15 mol%) could evidently promote this amidation.HOAc and Li2CO3 as co-additivesmake the yield increase to 68% (entries 3-7). The solvent also played a crucial role. Among the solvents tested (DCE,THF,1,4-dioxane,DMA,t-AmylOH and acetonitrile),DCE was proved to be the best for this transformation (entries 7-12). It is noteworthy that reaction temperature was critical for this transformation as well. Lower reaction temperatures resulted in decreasing reactivity. The yields were gradually improved to 95% by continuous increasing to 80 8C (entries 6,13- 16). Notably,this catalytic system also provided satisfactory yields even with a lower loading of iridium catalyst (1mol%) (entry 17). Furthermore,we examined various silver salt,AgNTf2 was proved to be the best (entries 18-22). In addition,no conversionwas observed with [RhCp*Cl2]2,[Ru(p-cymene)Cl2]2 or Pd(OAc)2 as catalyst (entries 23-25).
| Table 1 Optimization of various reaction parameters.a |
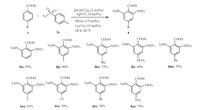
With the optimal reaction conditions in hand,we firstly investigated various ortho-benzoic acids 1 with para-methylphenylsulfonyl azide (2a) (Scheme 1). Benzoic acids with weakly electron-withdrawing and electron-donating groups at ortho position could be smoothly converted to the target products in excellent yields (3aa-3da,Scheme 1). Deficient sulfonyl azide,such as 2-nitrobenzoic acids (3ea),behaved less active. For metasubstituted benzoic acids,there are two different C-H bonds could be aminated. The electron-donating substituents (OMe) promoted the reaction producing the di-amidation products (3fa). For metanitrobenzoic acid only gave mono-amidated product because of its lower activity (3ga). meta-Bromobenzoic acid (1h) could generated isolatable mono- and di-amidation products 3ha and 4ha in totally excellent yield.

|
Download:
|
|
Scheme 1.Amidation of benzoic acids ( |
|
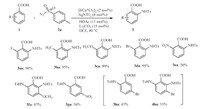
The scope of sulfonyl azides 2 was then examined in the amidation of 2-methylbenzoic acid (1b) (Scheme 2). Various functional groups,such as F,Cl and NO2,were well tolerated under the standard reaction conditions. Benzenesulfonyl azide (2b) resulted in the target product 3bb in excellent yield. For parasubstituted benzenesulfonyl azides,electron-donating substituents (3ba,3bc) promoted the reaction much more rapidly than the electron-withdrawing groups (3bd,2bf). Methyl substituent at ortho-position of the arene ring (3bg) led to a much lower yield than the meta-methyl benzenesulfonyl azide (3bh) due to the steric hindrance. Moreover,alkane sulfonyl azides 3bi and 3bj were readily amidated under the standard reaction conditions.
|
Download:
|
|
Scheme 2.Amidation of benzoic acid ( |
|
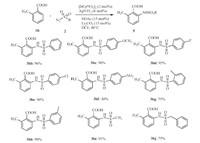
Next,we explored the generality and scope of para-substituted benzoic acids. As shownin Scheme 3 (1j-1q),di-amidated products,rather than mono-amidation,were consistently obtained in high yields under the standard reaction conditions,which are complementary to previous directed ortho-amidation reactions. Benzoic acids (4i) resulted in the target product 4ia in 95% yield. Electrondonating benzoic acid such as -Me,-tBu,-OCH3 and -Ph (4j,4k,4l and 4m) provided 90%,73%,60%,55%,respectively. In addition,benzoic acids bearing the halogen groups,such as,-F,-Cl,-Br (4n,4o,4p) and strong electron-withdrawing group -NO2 (1q) at the para position performed smoothly as well,delivering the desired products 4na,4oa,4pa,4qa in 92%,70%,70% and 79% yields,respectively. Good tolerance of large scope functional group made this reaction particularly attractive for increasing the molecular complexity by various chemical transformations.
|
Download:
|
|
Scheme 3.Amidation of para-benzoic acids ( |
|
According to the literatures [11, 12],a plausible mechanistic pathway for this Ir(III)-catalyzed amidation reaction was proposed in Scheme 4. Initially,a combination of AcOH and Li2CO3 in situ generated lithiumacetate and lithiumbicarbonate.Then,acetate ion and AgNTf2 prompted neutral dimeric iridium precursor to convert into its monomeric A,which dissociated the acetate ligand and combined with benzoic acid to form species B. Subsequently,iridium species induced C-H activation,leading to the formation of 5- or 6-membered metacyclic intermediate C and coordination of the azide to C formed the intermediate D. Finally,the desired amidated product 3 was generated with the formation of C-N bond and the release of N2.
|
Download:
|
| Scheme 4.Proposed reaction mechanism. | |
Insummary,we have developed an iridium-catalyzed direct C-H amidation of weakly coordinating benzoic acids with sulfonyl azides. The protocol exhibited a broad substrate scope and proceeded under mild conditions with excellent functional group tolerance. Carboxylic acids are more available compared to other traditional coupling reagents and easy to save,the process of carboxylic acid-directed C-H amination herein has a great significance on synthetic organic products and medicinal intermediates.
AcknowledgmentThis work was supported by Minjiang Scholar Program (No. 10BS216),Xiamen Southern Oceanographic Center (No. 13GYY003NF16) and Huaqiao University.
Appendix A. Supplementary dataSupplementarymaterial related to this article canbe found,inthe online version,at http://dx.doi.org/10.1016/j.cclet.2015.08.009.
| [1] | F. Ullmann, Ueber eine neue Darstellungsweise von Phenylathersalicylsaure, Ber. Dtsch. Chem. Ges. 37 (1904) 853-854. |
| [2] | P.E. Fanta, The ullmann synthesis of biaryls, Chem. Rev. 38 (1946) 139-196. |
| [3] | J.F. Hartwig, Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides, Acc. Chem. Res. 41 (2008) 1534-1544. |
| [4] | D.S. Surry, S.L. Buchwald, Biaryl phosphane ligands in palladium-catalyzed amination, Angew. Chem. Int. Ed. 47 (2008) 6338-6361. |
| [5] | H.Y. Song, D. Chen, C. Pi, X.L. Cui, Y.J. Wu, Palladium(II)-catalyzed direct regioselectively oxidative acylation of azobenzenes with toluene derivatives, J. Org. Chem. 79 (2014) 2955-2962. |
| [6] | Z.Y. Wu, H.Y. Song, X.L. Cui, et al., Sulfonylation of quinoline N-Oxides with aryl sulfonyl chlorides via copper-catalyzed C-H bonds activation, Org. Lett. 15 (2013) 1270-1273. |
| [7] | C.W. Zhu, M.L. Yi, D.H. Wei, et al., Copper-catalyzed direct amination of quinoline N-Oxides via C-H bond activation under mild conditions, Org. Lett. 16 (2014) 1840-1843. |
| [8] | G. Shan, G.Y. Huang, Y. Rao, H. Zhang, Palladium-catalyzed ortho-selective C-H bond chlorination of aromatic ketones, Chin. Chem. Lett. (2015), http://dx.doi.org/ 10.1016/j.cclet.2015.07.011. |
| [9] | T.M. Figg, S. Park, J. Park, S. Chang, Comparative investigations of Cp*-based group 9 metal-catalyzed direct C-H amination of benzamides, Organometallics 33 (2014) 4076-4085. |
| [10] | Q.Z. Zheng, Y.F. Liang, C. Qin, N. Jiao, Ru(II)-catalyzed intermolecular C-H amidation of weakly coordinating ketones, Chem. Commun. 49 (2013) 5654-5656. |
| [11] | J. Kim, S. Chang, Iridium-catalyzed direct C-H amidation with weakly coordinating carbonyl directing groups under mild conditions, Angew. Chem. Int. Ed. 53 (2014) 2203-2207. |
| [12] | J. Kim, J. Kim, S. Chang, Ruthenium-catalyzed direct C-H amidation of arenes including weakly coordinating aromatic ketones, Chem. Eur. J. 19 (2013) 7328-7333. |
| [13] | D. Lee, Y. Kim, S. Chang, Iridium-catalyzed direct arene C-H bond amidation with sulfonyl- and aryl azides, J. Org. Chem. 78 (2013) 11102-11109. |
| [14] | K. Shin, Y. Baek, S. Chang, Direct C-H amination of arenes with alkyl azides under rhodium catalysis, Angew. Chem. Int. Ed. 52 (2013) 8031-8036. |
| [15] | J. Ryu, K. Shin, S.H. Park, J.Y. Kim, S. Chang, Rhodium-catalyzed direct C-H amination of benzamides with aryl azides: a synthetic route to diarylamines, Angew. Chem. Int. Ed. 51 (2012) 9904-9908. |
| [16] | J.Y. Kim, S.H. Park, J. Ryu, et al., Rhodium-catalyzed intermolecular amidation of arenes with sulfonyl azides via chelation-assisted C-H bond activation, J. Am. Chem. Soc. 134 (2012) 9110-9113. |
| [17] | T. Kang, Y. Kim, D. Lee, Z. Wang, S. Chang, Iridium-catalyzed intermolecular amidation of sp3 C-H bonds: late-stage functionalization of an unactivated methyl group, J. Am. Chem. Soc. 136 (2014) 4141-4144. |
| [18] | H. Hwang, J. Kim, J. Jeong, S. Chang, Regioselective introduction of heteroatoms at the C-8 position of quinoline N-Oxides: remote C-H activation using N-Oxide as a stepping stone, J. Am. Chem. Soc. 136 (2014) 10770-10776. |
| [19] | K. Shin, S. Chang, Iridium(III)-catalyzed direct C-7 amination of indolines with organic azides, J. Org. Chem. 79 (2014) 12197-12204. |
| [20] | J. Ryu, K. Shin, S.H. Park, J.Y. Kim, S. Chang, Rhodium-catalyzed direct C-H amination of benzamides with aryl azides: a synthetic route to diarylamines, Angew. Chem. Int. Ed. 124 (2012) 10042-10046. |
| [21] | J. Ryu, J. Kwak, K. Shin, D. Lee, S. Chang, Ir(III)-catalyzed mild C-H amidation of arenes and alkenes: an efficient usage of acyl azides as the nitrogen source, J. Am. Chem. Soc. 135 (2013) 12861-12868. |
| [22] | M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art, M. Nomura, Oxidative cross-coupling of N-(2'-Phenylphenyl)benzene-sulfonamides or benzoic and naphthoic acids with alkenes using a palladium-copper catalyst system under air, J. Org. Chem. 63 (1998) 5211-5215. |
| [23] | H.A. Chiong, Q.N. Pham, O. Daugulis, Two methods for direct ortho-arylation of benzoic acids, J. Am. Chem. Soc. 129 (2007) 9879-9884. |
| [24] | R. Giri, N. Maugel, J.J. Li, D.H. Wang, S.P. Breazzano, L.B. Saunders, J.Q. Yu, Palladium-catalyzed methylation and arylation of sp2 and sp3 C-H bonds in simple carboxylic acids, J. Am. Chem. Soc. 129 (2007) 3510-3511. |
| [25] | D.H. Wang, T.S. Mei, J.Q. Yu, Versatile Pd(II)-catalyzed C-H activation/aryl- aryl coupling of benzoic and phenyl acetic acids, J. Am. Chem. Soc. 130 (2008) 17676-17677. |
| [26] | T.S. Mei, R. Giri, N. Maugel, J.Q. Yu, Pd(II)-catalyzed monoselective ortho halogenation of C-H bonds assisted by counter cations: a complementary method to directed ortho lithiation, Angew. Chem. Int. Ed. 47 (2008) 5215-5219. |
| [27] | Y.H. Zhang, J.Q. Yu, Pd(II)-catalyzed hydroxylation of arenes with 1 atm of O2 or air, J. Am. Chem. Soc. 131 (2009) 14654-14655. |
| [28] | Y.H. Zhang, B.F. Shi, J.Q. Yu, Palladium(II)-catalyzed ortho alkylation of benzoic acids with alkyl halides, Angew. Chem. Int. Ed. 48 (2009) 6097-6100. |
| [29] | K.M. Engle, D.H. Wang, J.Q. Yu, Constructing multiply substituted arenes using sequential palladium(II)-catalyzed C-H olefination, Angew. Chem. Int. Ed. 49 (2010) 6169-6173. |
| [30] | T.S. Mei, D.H. Wang, J.Q. Yu, Expedient drug synthesis and diversification via ortho-C-H iodination using recyclable PdI2 as the precatalyst, Org. Lett. 14 (2010) 3140-3143. |
| [31] | K.M. Engle, P.S. Thuy-Boun, M. Dang, J.Q. Yu, Ligand-accelerated cross-coupling of C(sp2)-H bonds with arylboron reagents, J. Am. Chem. Soc. 133 (2011) 18183-18193. |
| [32] | D. Lee, S. Chang, Direct C-H amidation of benzoic acids to introduce metaand para-amino groups by tandem decarboxylation, Chem. Eur. J. 21 (2015) 5364-5368. |
| [33] | M.R. Yadav, R.K. Rit, A.K. Sahoo, Sulfoximine directed intermolecular o-C-H amidation of arenes with sulfonyl azides, Org. Lett. 7 (2013) 1638-1641. |
| [34] | S. Doungsoongnuen, A. Worachartcheewan, R. Pingaew, et al., Investigation on biological activities of anthranilic acid sulfonamide analogs, EXCLI J. 10 (2011) 155-161. |
 2015, Vol.26
2015, Vol.26