2 State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin 300071, China;
3 Chongqing Key Laboratory for Advanced Materials & Technologies of Clean Energies, Chongqing 400715, China
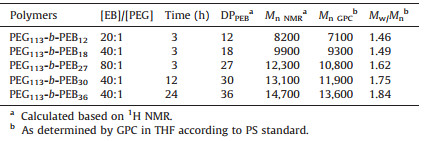
Amphiphilic copolymers with hydrophobic blocks and hydrophilic blocks have potential as drug delivery vehicles due to their ability to assemble into a variety of supramolecular aggregate structures including core-shell micelles,filomicelles,and vesicles [1, 2, 3]. As of this date,although the synthesis of amphiphilic block copolymers has been extensively reported for biomedical applications,most of the interest in hydrophobic blocks still focused on poly(ε-caprolactone) (PCL),or poly(lactic acid) (PLA) [4, 5, 6]. Polyesters such as PCL and PLA are an attractive choice as a core-forming block because they are considered biocompatible and biodegradable. More importantly,due to their hydrophobicity,such core-forming blocks are attractive for drug delivery purposes because of their ability to solubilize large amounts of poorly water-soluble drugs. In order to provide promising drug carrier candidates for clinical applications in delivery systems,it is highly advantageous to expand the hydrophobic block of amphiphilic copolymers. Ethylene brassylate is a 17 member ring lactone commercially available and less expensive than lactide,ε-caprolactone and other macrolactones [7]. Interestingly, poly(ethylene brassylate) (PEB) is a semi-crystalline polyester similar to PCL [8]. In this work,amphiphilic copolymers based on the PEB hydrophobic block were first synthesized through ringopening polymerization (ROP) of ethylene brassylate. The structure and synthesis routes of amphiphilic block copolymers with poly(ethylene glycol) (PEG) and PEB are illustrated in Fig. 1. Additionally,the self-assembled morphology of PEG-b-PEB and the drug release behavior of nanoparticles loaded hydrophobic doxorubicin (DOX) were investigated.
|
Download:
|
| Fig. 1. Synthesis of amphiphilic PEG-b-PEB. | |
Ethylene brassylate (EB) (98%) and methoxy poly(ethylene glycol) (PEG) with an average molecular weight of 5000 were purchased from Aldrich and used without further treatment. The 1,5,7-triazabicyclo[4.4.0]-dec-5-ene (TBD) (98%) was obtained from Energy Chemical and used as obtained. All other chemicals and solvents were purchased from commercial sources and used without further purification.
2.2. Synthesis and self-assembly of PEG-b-PEB0.39 g of PEG (0.078 mmol) and 0.0108 g of TBD (0.078 mmol) were dissolved in 5 mL of toluene in a 25 mL dry flask under nitrogen atmosphere. Then,0.9 mL of EB (3.3 mmol) was added to the solution via syringe. The mixture was stirred at 80 ℃ over the appropriate reaction time. The crude polymer was dissolved in dichloromethane,then precipitated in cold diethyl ether and dried under vacuum at 30 °C. According to similar procedures,other block copolymers with varying EB contents were also synthesized and their structural parameters summarized in Table 1.
| Table 1 Experimental conditions and characterization results of PEG-b-PEB polymers. |
Self-assembly of PEG-b-PEB,DOX encapsulation and in vitro drug release were completed according to our previous reported procedure [9].
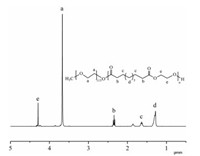
3. Results and discussionThe amphiphilic block copolymer PEG-b-PEB was synthesized utilizing methoxy poly(ethylene glycol) as the macroinitiator for the ROP of ethylene brassylate (EB) in the presence of 1,5,7- triazabicyclo[4.4.0]dec-5-ene (TBD) as an organic catalyst. Table 1 represents an overview of the reaction conditions and the results of the different ROP polymerization that were performed. The EB/PEG molar ratios and reaction times were adjusted to achieve different chain lengths of PEB. For an EB/PEG molar ratio of 20:1,the number-average molecular weight (Mn) and polydispersity index (Mw/Mn) of PEG-b-PEB measured by GPC were 7100 g/mol and 1.46,respectively. With an increase in molar ratio of EB to PEG from 40:1 to 80:1,Mn of PEG-b-PEB increased from 9300 to 10,800 g/mol. To the same molar ratios (40:1),PEG-b-PEB of Mn ranging between 9300 and 13,600 g/mol and Mw/Mn between 1.49 and 1.84 were obtained when reaction time increased from 3 h to 24 h. As can be seen from Table 1,the NMR and GPC molecular weights were in good agreement. Successful synthesis of PEG-bPEB was further confirmed by 1H NMR. A representative 1H NMR spectrum of PEG113-b-PEB36 is provided in Fig. 2. It clearly shows that besides the major proton signal of PEG chains,there were additional signals (labeled b-e) of the hydrophobic block PEB. The degree of polymerization (DP) of EB was evaluated by comparing the integration of signals at 3.64 ppm for CH2 of PEG (labeled a) and the triplet at 2.43 ppm for CH2 of PEB (labeled b). These observations indicated the successful preparation of PEG-b-PEB. Furthermore,the thermal transitions of the PEB homopolymer were investigated by differential scanning calorimetry (DSC). As shown in the DSC (Fig. S1 in Supporting information),PEB is a semicrystalline,long-chain polyester with a Tg of -22.6 and melting temperature (Tm) of 46.5 ℃ similar to short-chain polyesters,such as PCL with a Tm of 30.7 ℃.
|
Download:
|
| Fig. 2. 1H NMR spectrum of PEG113-b-PEB36. | |
The other objectives of this work were: (1) to demonstrate the stability and drug reservoir capabilities of the novel PEG-b-PEB micelles based on the PEB hydrophobic block under physiological conditions and (2) to also study their performance as a drug delivery carrier. It is well known that PEB homopolymer is hydrophobic,whereas PEG is water-soluble at room temperature. Thus,PEG-b-PEB micelles with a core PEB block and a shell of PEG were formed by adding the polymer solution in DMF into 10-folds of water at 25 ℃. The critical micelle concentration (CMC) values verified the micelles formation. As shown in Fig. S2 in Supporting information,as the concentration of the relevant polymers increased,the fluorescence intensity of pyrene was abruptly increased at a certain concentration. The low CMC values of PEG113-b-PEB36 (2.2 × 10 -3 mg/mL) and PEG113-b-PEB12 (3.4 × 10 -3 mg/mL) enable the micelles to remain stable in a dilute aqueous environment,such as body fluids and blood. This is also necessary for an anticancer drug delivery system to guarantee the expected effect in the targeted tissues and limit the prior degradation in the blood circulation.
The morphologies of the self-assembled aggregates of PEG113-b-PEB36 and PEG113-b-PEB12 copolymers were investigated by transmission electron microscopy (TEM) and dynamic light scattering (DLS) as shown in Fig. 3. Discher and Eisenberg [10] have presented interesting research and developed a relationship between the block copolymer composition and the self-assembled morphologies. They found that the self-assembled morphology was most simply a reflection of the hydrophilic-to-hydrophobic ratio. In their theory,spherical micelles are expected for block polymers with hydrophilic mass fraction ( f) greater than 45%, whereas block copolymers with lower hydrophilic mass fraction ( f) typically self-assemble into vesicles. A number of reviews have also been published describing the relationship between the hydrophilic mass fraction ( f) and the resulting solution morphologies [11, 12]. Spherical vesicles ranging from 120 nm to 170 nm were observed,as shown in Fig. 3a,when the weight fraction of the PEG segment is 37% for PEG113-b-PEB36. With the decreasing of the weight fraction of the PEB segment (PEG weight fraction 70%), PEG113-b-PEB12 preferred to form spherical micelles with the number average mean diameters ranging from 80 nm to 120 nm (Fig. 3b). From the DLS experiments (Fig. 3c),the average diameters of nanoparticles were about 169 nm for PEG113-b-PEB36 and 126 nm for PEG113-b-PEB12,respectively. It is noteworthy that the size of the micelles determined by DLS is larger than observed by TEM,which is attributed to the dehydration of micelles caused by solvent evaporation and therefore the collapse and shrinkage of micelles in TEM experiment.

|
Download:
|
| Fig. 3. TEM images of (a) PEG113-b-PEB36 vesicles and (b) PEG113-b-PEB36 micelles. (c) Plots of hydrodynamic diameter (Dh) of PEG113-b-PEB36 vesicles and PEG113-b-PEB36micelles, respectively. (d) In vitro release of DOX from PEG113-b-PEB36 vesicles and PEG113-b-PEB36 micelles, respectively. | |
Generally,DOX,one of the most potent anticancer drugs,is extensively used to treat different types of solid malignant tumors through interacting with DNA through interaction and inhibition of macromolecular biosynthesis [9]. To investigate the potential of novel diblock copolymer micelles based on self-assembly,DOX was selected to evaluate the drug loading and release properties. Loadings of DOX into PEG113-b-PEB36 vesicles and PEG113-b-PEB36 micelles were performed at a theoretical drug loading content (DLC) of 4.3 and 6.1 wt% with DOX loading efficiencies of 48 and 59%,respectively. The in vitro release profiles of PEG113-b-PEB36 and PEG113-b-PEB36 were evaluated at 37 ℃ in buffers (pH 7.4). As illustrated in Fig. 3d,approximately 45 and 35% of DOX was released in 24 h from PEG113-b-PEB36 vesicles and PEG113-b-PEB36 micelles,respectively. From Fig. 3d,the DOX release behaviors of the carriers and their comparison had similar typical two-phase release profiles. In the first stage,about 25%-37% DOX encapsulated into vesicles or micelles released within 10 h,followed by a sustained and slow release over a prolonged time. It is expected that most of DOX remains in the vesicles or micelles cores for a considerable time when these vesicles or micelles circulate in the plasma at normal physiological conditions. Under the same conditions,DOX-loaded PEG113-b-PEB36 vesicles displayed somewhat faster drug release than DOX-loaded PEG113-b-PEB36 micelles,which is most likely due to the thinner hydrophobic layer of the vesicles.
4. ConclusionIn summary,a novel biodegradable amphiphilic diblock copolymer PEG-b-PEB was designed and prepared for the delivery of the anticancer drug,DOX. The EB/PEG molar ratios and reaction time were adjusted to achieve amphiphilic PEG-b-PEB copolymers with different chain lengths of PEB. 1H NMR analysis confirmed the designed structures. These copolymers could self-assemble into the micelles and vesicles in aqueous solution,which can be controlled by the length of the hydrophobic PEB block. The DOXloaded micelles and vesicles of the amphiphilic diblock copolymers exhibited controllable drug-release behavior. Therefore,a promising strategy to synthesize novel biocompatible and biodegradable copolymers based on the hydrophobic PEB block as potential nanodrug carriers in a delivery system has been developed.
AcknowledgmentsThis work is financially supported by the Open Fund of State Key Laboratory of Medicinal Chemical Biology (Nankai University) under grant 20140523 and the Fundamental Research Funds for the Central Universities (Nos. SWU 113075 and XDJK2014B015)
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.05.050.
| [1] | Z.L.Tyrrell,Y.Q.Shen,M.Radosz,Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers,Prog.Polym.Sci.35(2010)1128-1143. |
| [2] | J.C.Chen,M.Z.Liu,H.H.Gong,Y.J.Huang,C.Chen,Synthesis and self-assembly of thermoresponsive PEG-b-PNIPAM-b-PCL ABC triblock copolymer through the combination of atom transfer radical polymerization,ring-opening polymerization,and click chemistry,J.Phys.Chem.B 115(2011)14947-14955. |
| [3] | Y.Zhou,H.Li,Y.W.Yang,Controlled drug delivery systems based on calixarenes,Chin.Chem.Lett.(2015),http://dx.doi.org/10.1016/j.cclet.2015.01.038. |
| [4] | X.B.Zhao,P.Liu,Reduction-responsive core-shell-corona micelles based on triblock copolymers:novel synthetic strategy,characterization,and application as a tumor microenvironment-responsive drug delivery system,ACS Appl.Mater.Interfaces 7(2015)166-174. |
| [5] | J.B.Song,Z.Fang,C.X.Wang,et al.,Photolabile plasmonic vesicles assembled from amphiphilic gold nanoparticles for remote-controlled traceable drug delivery,Nanoscale 5(2013)5816-5824. |
| [6] | G.H.Zhang,R.X.Hou,D.X.Zhan,et al.,Fabrication of hollow porous PLGA microspheres for controlled protein release and promotion of cell compatibility,Chin.Chem.Lett.24(2013)710-714. |
| [7] | A.Pascual,H.Sardon,A.Veloso,F.Ruipérez,D.Mecerreyes,Organocatalyzed synthesis of aliphatic polyesters from ethylene brassylate:a cheap and renewable macrolactone,ACS Macro Lett.3(2014)849-853. |
| [8] | A.Pascual,H.Sardon,F.Ruiperez,et al.,Experimental and computational studies of ring-opening polymerization of ethylene brassylate macrolactone and copolymerization with ε-caprolactone and TBD-guanidine organic catalyst,J.Polym.Sci.A:Polym.Chem.53(2015)552-561. |
| [9] | J.C.Chen,M.Z.Liu,Amphiphilic block copolymer micelles with fluorescence as nano-carriers for doxorubicin delivery,RSC Adv.4(2014)9684-9692. |
| [10] | D.E.Discher,A.Eisenberg,Polymer vesicles,Science 297(2002)967-973. |
| [11] | F.H.Meng,Z.Y.Zhong,J.Feijen,Stimuli-responsive polymersomes for programmed drug delivery,Biomacromolecules 10(2009)197-209. |
| [12] | R.P.Brinkhuis,F.P.J.T.Rutjes,J.C.M.van Hest,Polymeric vesicles in biomedical applications,Polym.Chem.2(2011)1449-1462. |
 2015, Vol.26
2015, Vol.26