In the last two decades,multicomponent reactions (MCRs) have been widely used in organic synthesis due to their efficacy for the generation of heterocyclic compounds in a single synthetic step [1]. These reactions represent very powerful chemical technology procedures from both economical and synthetic points of view and they are one of the most influential techniques in green chemistry, industrial chemistry and the modern drug discovery process [2]. Therefore,the discovery of novel MCRs is an interesting topic for synthetic chemistry researchers.
Recently,solid-supported heterogeneous catalysts have gained considerable interest in organic synthesis because of their unique properties such as high efficiency due to more surface area,more stability,reusability,low toxicity and ease of handling [3, 4, 5, 6, 7, 8]. We have recently reported nano titania-supported sulfonic acid (n-TSA) [9],as an effective heterogeneous acidic nanocatalyst for the promotion of a wide range of organic reactions as for its Lewis and Bronsted acidity features. Ease of preparation,simple work-up procedures and improved product yields,facile purification, shorter reaction times,mild reaction conditions and recyclability of the catalyst are the main superiorities of n-TSA.
Pyridine ring systems,particularly 2,4,6-triarylpyridines represent an important class of heterocyclic compounds because of their unique position in medicinal chemistry [10, 11]. Moreover,they are prominent synthons in supramolecular chemistry,with their π-stacking ability along with directional H-bonding capacity [12, 13]. In addition,the excellent thermal stabilities of these pyridines have instigated a growing interest for their use as monomeric building blocks in thin films and organometallic polymers [14].
Since,Krohnke’s original report on the synthesis of 2,4,6- triarylpyridines [15],there has been a plethora of research targeting their syntheses [16, 17]. Recently,the synthetic approach to 2,4,6-triarylpyridines involves cyclo-condensation reaction of acetophenones,benzaldehydes and ammonium acetate in the presence of different catalysts under heating condition or sonication and microwave irradiation [18, 19, 20, 21, 22].
Wide variety of 2,4,6-triarylpyridine chemical and pharmacological applications,encouraged us to develop and validate an efficient protocol for their synthesis. Herein,we have reported nano titania-supported sulfonic acid catalyzed synthesis of 2,4,6- triarylpyridines under solvent-free conditions.
2.ExperimentalChemicals were purchased from the Merck chemical companies. Thin-layer chromatography (TLC) on commercial plates of silica gel 60 F254 was used to monitor the progress of reactions. The products were characterized by FT-IR spectra,1H NMR and 13C NMR. FT-IR spectra were recorded on Shimadzu FT-IR 8400 instrument. 1H NMR and 13C NMR spectra were recorded on Bruker Advance Spectrometer 300 MHz and 75 MHz,respectively,using CDCl3 as solvent. The chemical shifts are expressed in parts per million (ppm) and tetramethylsilane (TMS) was used as an internal reference. Melting points were recorded on a THERMO SCIENTIFIC 9100 apparatus.
2.1. General procedure for the synthesis of catalyst (n-TSA)Chlorosulfonic acid (1 mL,15 mmol) was added dropwise to a suspension of powdered nano TiO2 (4 g) in dry CH2Cl2 (20 mL) over a period of 30 min while the mixture was stirred slowly in an ice bath. The mixture was stirred for 30 min at room temperature until HCl evolution was seized. Then,the CH2Cl2 was removed under reduced pressure and the solid powder was washed with ethanol (10 mL) and dried at 70 ℃. n-TSA was obtained as a white solid powder [9].
The mmol of H+ per gram of catalyst was also evaluated. For this purpose,the surface acidic protons of nano-TiO2-SO3H (100 mg) were ion-exchanged with a saturated solution of NaCl (10 mL) by sonicating. This process was repeated twice more,yielding 30 mL of proton-exchanged brine solution. Therefore,to determine the loading of acid sites on the synthesized catalyst the obtained solution titrated with NaOH (0.1 mol/L) solution in the presence of phenol red indicator solution or pH meter. The amounts of acidic protons found to be 4.5 mmol g-1 of n-TSA. This finding means the effective density of the acid sites.
The catalyst was characterized in our previously published paper by the X-ray diffraction (XRD),scanning electron microscopy (SEM),FT-IR spectroscopy,thermal gravimetric analysis (TGA) and the acid strength of n-TSA was determined by the Hammett acidic function too [9].
2.2. General procedure for the synthesis of 2,4,6-triarylpyridine derivativesA mixture of acetophenones (2 mmol),aromatic aldehydes (1 mmol),ammonium acetate (1.5 mmol) and 0.009 g of n-TSA was stirred at 110 ℃ under solvent-free condition for an appropriate time. After completion of the reaction (monitored by TLC),the reaction mixture was cooled,eluted with hot ethanol (2 mL) and was centrifuged to separate the catalyst. The title compounds were obtained in their crystalline forms by recrystallization of ethanol solution.
2,6-Bis(4-bromophenyl)-4-(p-tolyl)pyridine (5c): Light green crystal; mp: 236-240 ℃; IR (KBr,cm-1) υmax: 3028,1601,1577, 1516,1489; 1H NMR (300 MHz,CDC13): δ 2.46 (s,3H),7.33 (d,2H, J = 8.12 Hz),7.60 (m,6H),7.80 (m,2H),8.05 (dd,4H,J = 7.66, 4.02 Hz); 13C NMR (75 MHz,CDCl3): δ 21.3,116.8,123.6,126.9, 128.6,129.9,131.8,135.6,138.2,139.3,150.3,156.3.
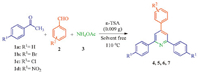
3. Results and discussionConsidering our researches toward evaluating the catalytic activity of n-TSA for the synthesis of organic compounds [9, 23],we have investigated the synthesis of 2,4,6-triarylpyridines (4-7) via one-pot,three-component cyclo-condensation reaction of acetophenones (1),aldehydes (2) and ammonium acetate (3) under solvent-free conditions (Scheme 1).
|
Download:
|
| Scheme. 1.Synthesis of 2,4,6-triarylpyridine derivatives by n-TSA. | |
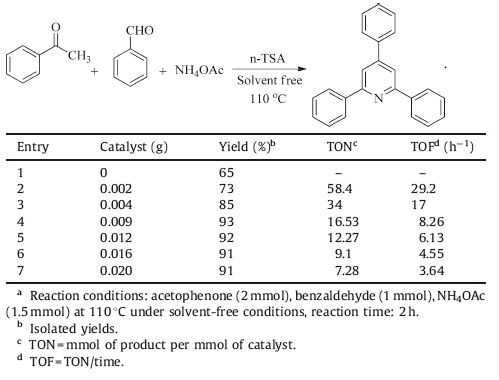
To choose the most appropriate condition in this reaction and to understand the influence of different variables,several experiments were studied. Initially,we optimized the amount of catalyst in order to find the required catalyst load. The obtained results are listed in Table 1.
| Table 1Optimum amount of catalyst on the synthesis of 2,4,6-triphenylpyridine.a |
As indicated in Table 1,the best yield was obtained in the presence of 0.009 g catalyst,which is enough to carry out the reaction efficiently (Table 1,entry 4).
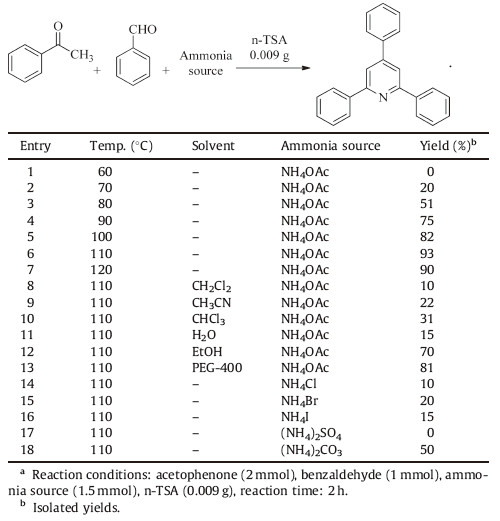
In the next step,the effect of temperature,solvent and ammonia source was examined over the reaction and the obtained results are shown in Table 2.
| Table 2Optimum condition on the synthesis of 2,4,6-triphenylpyridine.a |
As can be seen in Table 2,the product yield increased as the reaction temperature was raised at 110 ℃ and the product was obtained in high yield which was considered as the optimum temperature (Table 2,entry 6),but at higher temperature the yield was decreased (Table 2,entry 7) due to side reactions monitored by TLC. On the other hand,neither polar nor non-polar solvents are suitable for this reaction (Table 2,entries 8-13) and the best yield was obtained under solvent-free condition (Table 2,entry 6), which is due to the appropriate interaction between the catalyst and solvent. Eventually,the effect of various ammonia sources on yield of the product was shown that the maximum yield was obtained for NH4OAc (Table 2,entry 6),while other ammonia sources required longer reaction times and produced lower yields (Table 2,entries 14-18).
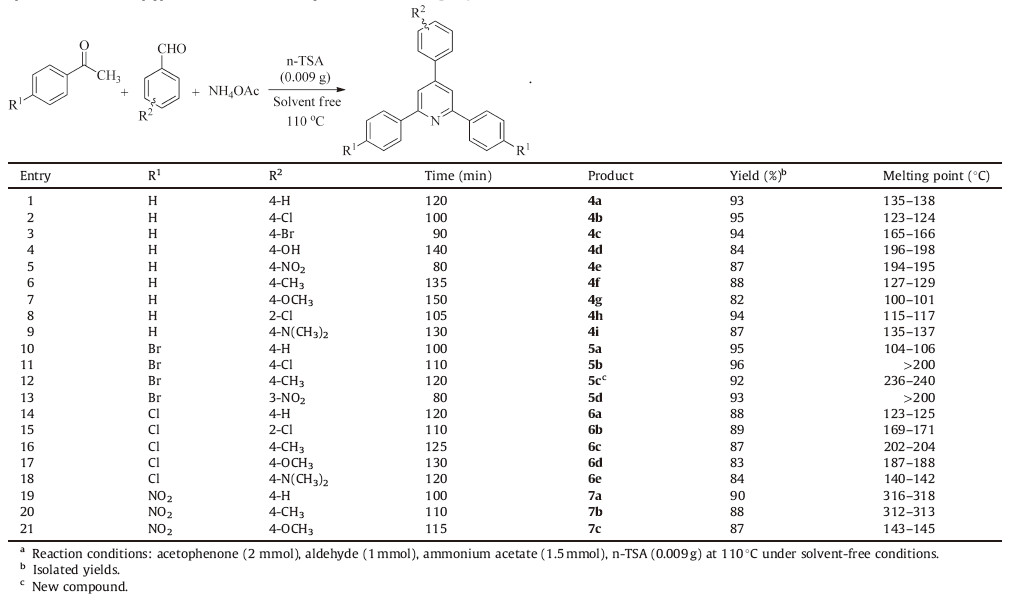
To develop the scope of the reaction,a wide range of aldehydes and acetophenones was subjected to n-TSA-catalyzed reaction under the optimized conditions (Table 3).
| Table 3Synthesis of 2,4,6-triarylpyridine derivatives in the presence of nano-TiO2-SO3H.a |
In all cases,aromatic aldehydes with substituents carrying either electron-donating or electron-withdrawing groups reacted successfully and gave the products in good yields (Table 3). It was found that aromatic aldehydes with electron-withdrawing groups(Table 3,entries 2,3,5,8,11,13,15) reacted faster than those with electron-donating groups (Table 3,entries 4,6,7,9,12,16-18,20, 21),as would be expected [24, 25]. These results justified one more time the efficiency of n-TSA.
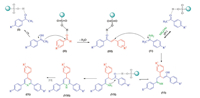
The proposed mechanism for the formation of 2,4,6-triarylpyridines is depicted in Scheme 2. The reaction involves four major stages: Aldol condensation,Michael addition,cyclization,and oxidation. Initially,acetophenone (I) in the presence of nano-TiO2- SO3H is converted into its enol form which gives nucleophilic addition to the arylaldehyde (II),which is activated by the catalyst, to afford aldol condensation product (III). Then,a molecule of acetophenone is condensed with an ammonia source (IV) and enamine (V) is formed. SO3H group of n-TSA enhances the electron deficiency on carbonyl group of (III) which easily could be attacked by (V) in a Michael addition reaction to afford intermediate (VI). The following cyclisation provides dihydropyridine (VIII). Finally, air oxidation gives the final product (IX). As indicated in Scheme 2, the SO3H groups of n-TSA plays principal role in the catalytic activity of the catalyst and promotion of the reaction rate.
|
Download:
|
| Scheme. 2.Plausible mechanism for the synthesis of 2,4,6-triarylpyridines by n-TSA. | |
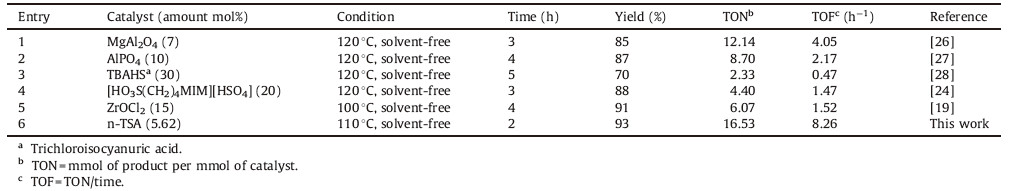
In order to exhibit the merit of the present work,our results are compared with some other previously reported studies in the Table 4.
| Table 4Comparison n-TSA with other reported catalysts in the synthesis of 2,4,6-triphenylpyridine. |
As shown in Table 4,n-TSA can act as an effective catalyst with respect to the reaction time,yield of the product,TON (turnover number) and TOF (turnover frequency). Moreover,it can be recovered and reused for several times.
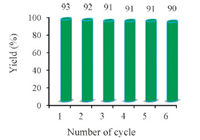
The principle advantage of the use of heterogeneous catalysts in organic transformations is their reusability. For this purpose,after completion of the reaction,the reaction mixture was cooled,eluted with hot ethanol (2 mL) and was centrifuged to filter the catalyst. The catalyst was then washed with ethanol,dried at 50 ℃ and used directly for the synthesis of 2,4,6-triphenylpyridine. The recovered n-TSA was reused for 6 consecutive reactions and obtained the yield 90%-93%. This indicates that n-TSA does not lose its activity and can be recyclable (Fig. 1).
|
Download:
|
| Fig. 1.Reusability of n-TSA for the synthesis of 2,4,6-triphenylpyridine. | |
The particle size of the recovered catalyst was confirmed by the scanning electron microscopy of the six-time reused catalyst,which clearly demonstrated the same particle size (spherical powder with average size of 35-60 nm) and high stability of the nano TiO2-based solid acid catalyst (Fig. 2).

|
Download:
|
| Fig. 2.SEM images of n-TSA before use (a) and after reuse six times (b). | |
4. Conclusion
In summary,an efficient and rapid route for the synthesis of 2,4,6-triarylpyridines under solvent-less conditions is reported. Operational simplicity,short reaction time,simple work-up without column chromatographic purification,reusability of the catalyst,tolerance of various functional groups,excellent yields of products and solvent free conditions are the advantages of this protocol. Moreover,one new compound is reported.
AcknowledgmentWe gratefully acknowledge the Faculty of Chemistry of Semnan University for supporting this work.
| [1] | R.W. Armstrong, A.P. Combs, P.A. Tempest, S.D. Brown, T.A. Keating, Multiplecomponent condensation strategies for combinatorial library synthesis, Acc. Chem. Res. 29(1996) 123-131. |
| [2] | Y.A. Ibrahim, H. Behbehani, M.R. Ibrahim, Efficient atom economic approaches towards macrocyclic crownamides via ring closure metathesis, Tetrahedron Lett. 43(2002) 4207-4210. |
| [3] | M. Sathishkumar, S. Nagarajan, P. Shanmugavelan, et al., One-pot regio/stereoselective synthesis of 2-iminothiazolidin-4-ones under solvent/scavenger-free conditions, Beilstein J. Org. Chem. 9(2013) 689-697. |
| [4] | R.S. Varma, Solvent-free organic syntheses using supported reagents and microwave irradiation, Green Chem. 1(1999) 43-55. |
| [5] | H. Naeimi, Z.S. Nazifi, Sulfonated diatomite as heterogeneous acidic nanoporous catalyst for synthesis of 14-aryl-14-H-dibenzo[a,j]xanthenes under green conditions, Appl. Catal. A 477(2014) 132-140. |
| [6] | P. Sivaguru, A. Lalitha, Ceric ammonium nitrate supported HY-zeolite:an efficient catalyst for the synthesis of 18-dioxo-octahydroxanthenes, Chin. Chem. Lett. 25(2014) 321-323. |
| [7] | P. Li, S. Regati, H.C. Huang, et al., A sulfonate-based Cu(I) metal-organic framework as a highly efficient and reusable catalyst for the synthesis of propargylamines under solvent-free conditions, Chin. Chem. Lett. 26(2015) 6-10. |
| [8] | S.R. Jetti,A. Bhatewara, T. Kadre, S. Jain, Silica-bonded N-propyl sulfamic acid as an efficient recyclable catalyst for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under heterogeneous conditions, Chin. Chem. Lett. 25(2014) 469-473. |
| [9] | S. Rahmani, A. Amoozadeh, E. Kolvari, Nano titania-supported sulfonic acid:an efficient and reusable catalyst for a range of organic reactions under solvent free conditions, Catal. Commun. 56(2014) 184-188. |
| [10] | I.J. Enyedy, S. Sakamuri, W.A. Zaman, K.M. Johnson, S. Wang, Pharmacophorebased discovery of substituted pyridines as novel dopamine transporter inhibitors, Bioorg. Med. Chem. Lett. 13(2003) 513-517. |
| [11] | B.Y. Kim, J.B. Ahn, H.W. Lee, et al., Synthesis and biological activity of novel substituted pyridines and purines containing 2,4-thiazolidinedione, Eur. J. Med. Chem. 39(2004) 433-447. |
| [12] | E.C. Constable, Metallodendrimers:metal ions as supramolecular glue, Chem. Commun. (1997) 1073-1080. |
| [13] | E.C. Constable, E.L. Dunphy, C.E. Housecroft, et al., Structural development of free or coordinated 40-(4-pyridyl)-2, 20:60,200-terpyridine ligands through N-alkylation:new strategies for metallamacrocycle formation, Chem. Eur. J. 12(2006) 4600-4610. |
| [14] | B.G.G. Lohmeijer, U.S. Schubert, Playing LEGO with macromolecules:design, synthesis, and self-organization with metal complexes, J. Polym. Sci. A:Polym. Chem. 41(2003) 1413-1427. |
| [15] | F. Krohnke, The specific synthesis of pyridines and oligopyridines, Synthesis 1(1976) 1-24. |
| [16] | G.W.V. Cave, C.L. Raston, Efficient synthesis of pyridines via a sequential solventless aldol condensation and Michael addition, J. Chem. Soc., Perkin Trans. 1(2001) 3258-3264. |
| [17] | K.T. Potts, M.J. Cipullo, P. Ralli, G. Theodoridis, Synthesis of 2,6-disubstituted pyridines, polypyridinyls, and annulated pyridines, J. Org. Chem. 47(1982) 3027-3038. |
| [18] | M. Wang, Z. Yang, Z. Song, Q. Wang, Three-component one-pot synthesis of 2,4,6-triarylpyridines without catalyst and solvent, J. Heterocycl. Chem. 52(2014) 907-910. |
| [19] | A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, et al., Synthesis of 2,4,6-triarylpyridines using ZroCl2 under solvent-free conditions, Synlett 25(2014) 193-196. |
| [20] | Z. Zarnegar, J. Safari, M. Borjian-borujeni, Ultrasound-mediated synthesis of 2,4,6-triaryl-pyridines using MgAl2O4 nanostructures, Chem. Heterocycl. Compd. (2015) 1-9. |
| [21] | M. Reza, M. Shafiee, R. Moloudi, M. Ghashang, ZnO nanopowder:an efficient catalyst for the preparation of 2,4,6-triaryl pyridines under solvent-free condition, APCBEE Proc. 1(2012) 221-225. |
| [22] | J. Safari, S. Gandomi-Ravandi, M. Borujeni, Green and solvent-free procedure for microwave-assisted synthesis of 2,4,6-triarylpyridines catalysed using MgAl2O4 nanocrystals, J. Chem. Sci. 125(2013) 1063-1070. |
| [23] | A. Amoozadeh, E. Tabrizian, S. Rahmani, Nano titania-supported sulfonic acid catalyzed synthesis of α,α'-bis(substituted-benzylidene)cycloalkanones and their xanthene derivatives under solvent-free conditions, C. R. Chim. 18(2015) 848-857. |
| [24] | A. Davoodnia, M. Bakavoli, R. Moloudi, N. Tavakoli-Hoseini, M. Khashi, Highly efficient, one-pot, solvent-free synthesis of 2,4,6-triarylpyridines using a Brønsted-acidic ionic liquid as reusable catalyst, Monatsh. Chem. Chem. Mon. 141(2010) 867-870. |
| [25] | K.S. Vellayan Kannan, Montmorillonite K10 clay catalyzed one pot synthesis of 2,4,6-trisubstituted pyridine under solvent free condition, Mod. Res. Catal. 2(2013) 42-46. |
| [26] | J. Safari, Z. Zarnegar, M. Borujeni, Mesoporous nanocrystalline MgAl2O4:a new heterogeneous catalyst for the synthesis of 2,4,6-triarylpyridines under solventfree conditions, Chem. Pap. 67(2013) 688-695. |
| [27] | P. Rajput, N.J.P. Subhashini, S. Raj, Synthesis of 2,4,6-triarylpyridines using AlPO4 under solvent-free conditions, J. Sci. Res. 2(2010) 337-342. |
| [28] | K.S. Reddy, R.B. Reddy, K. Mukkanti, G. Thota, G. Srinivasulu, Synthesis of 2,4,6-triarylpyridines using TBAHS as a catalyst, Rasayan J. Chem. 4(2011) 299-302. |
 2015, Vol.26
2015, Vol.26