b School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
Fluorinated surfactants are more efficient than traditional surfactants since they display high surface activity at low critical micelle concentration (cmc). Besides,fluorinated surfactants usually have high thermal and chemical stability [1, 2, 3]. They are usually composed of a perfluorinated chain and a hydrophilic group [4, 5, 6]. The most known fluorinated surfactants are PFOA (C7F15CO2H),APFO (ammonium perfluorooctanoate) and PFOS (C8F17SO3X,with X = K,Na,H). Up to now,fluorinated surfactants have been widely used in more than 200 applications [7] including soil and stain-repellents,plane hydraulic fluids,paints,coatings for clothing fabrics,electroplating,fire fighting foams,food packaging, petroleum,textile,etc. In addition PFOA is also frequently used as surfactant in aqueous media of polymerization of hydrophobic monomers,especially fluorinated monomers such as tetrafluoroethylene and other C2-C3 alkenes.
Apart from all the advantages and uses of PFOA and PFOS,they are listed under Stockholm convention on persistent organic pollutants (POPs) owing to their non-biodegradable wastage and toxic effects in the environment [8, 9, 10, 11, 12]. In fact,perfluoroalkyl substances have been detected worldwide in human blood/serum, with PFOS being the most prevalent compound in humans, followed by PFOA [13].
For these above reasons,EPA launched the PFOA Stewardship Program [14] to decrease the production of PFOA and PFOS to 95% by 2010 and to eliminate emissions and products content of these chemicals by 2015. This programhas gathered themost important manufactures of PFOA,PFOS and fluorinated polymers. Attempts to degrade PFOA and PFOS was suggested by Parsons et al. [15],but these authors demonstrated that the lack of mineralization is probably caused by the stability of the C-F bond although there are examples of microbially catalyzed defluorination reactions. So,it is necessary to develop novel structural fluorinated surfactants.
The objectives of thisminireviewconcern various strategies for synthesizing non-bioaccumulable alternatives to PFOA and PFOS. Three main alternative orientations are considered: (I) reducing the length of the perfluorocarbon chain,(II) introducing hetero atoms into the fluorocarbon chain,(III) introducing branch. In this minireview,we consider water-surfactants only,and not surfactants for supercritical CO2 in which usually a (per)fluorinated sequence is CO2-philic while other block is CO2-phobic [16]. 2. Strategies for synthesizing non-bioaccumulable alternatives to PFOA and PFOS 2.1. Reducing the length of the perfluorocarbon chain 2.1.1. PFBS
The 3M Company launched new products named Scotchgard Protectoro by replacing PFOS to PFBS (perfluorobutane sulfonate,C4F9SO3H) in 2003. Studies shown that PFBS has no significant persistent bioaccumulative and it can be excreted with the body’s metabolism in short time,besides,the degradation products are non-toxic. Later studies found that products made from PFBS have good waterproof and decontamination capabilities, but cannot reach the oil repellent level of products made from PFOS. 2.1.2. PFHS
Some companies launched new products by replacing PFOS to PFHS (perfluorohexane sulfonate,C6F13SO3H) after 2005. Indeed, these products were under big controversy. Studies have shown that PFHS was also very difficult to be degraded and its toxicity to some aquatic organisms was three to five times bigger than PFOS [17]. 2.1.3. C4 or C6 perfluorinated surfactants (the length of the perfluorocarbon chain is four or six)
Generally,compounds containing active functional groups, such as halogen [18],carboxylic acid [19],sulfonyl [20, 21, 22, 23, 24, 25, 26, 27], alcohol [28] and so on,are used as the starting material (Schemes 1-4).
|
Download:
|
| Scheme. 1. Surfactants derived from perfluoroalkyl iodide. | |
|
Download:
|
| Scheme. 2. Surfactants derived from perfluoroalkyl carboxylic acid. | |
|
Download:
|
|
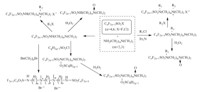
| Scheme. 3. Surfactants derived from perfluoroalkyl sulfonyl fluorid or perfluoroalkyl sulfonyl chloride. | ||
|
Download:
|
||
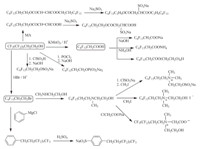
| Scheme. 4. Surfactants derived from fluorinated alkyl alcohol. | |||
The oil repellent performance of the fluorinated finishing agent containing C4 and C6 perfluorinated surfactants is worse than that of C8 perfluorinated surfactants. Besides,perfluorinated chain is difficult to synthesize. The methods reported are fluorination by electrolysis [29] and telomerization of fluoroolefin [30, 31, 32],etc. Fluorination by electrolysis uses HF as a main raw material which is inexpensive,but the method has many by-products. Telomerization of fluoroolefin usually generates a mixture of products with different chain length,therefore,strict control of reaction conditions is required. So,we should get out of perfluorinated alkane sulfonic acid or carboxylic development orientation to find other ways to replace PFOS. 2.2. Introducing hetero atoms into the fluorocarbon chain 2.2.1. Surfactants from oligo(hexafluoropropylene oxide)
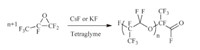
Oligo(hexafluoropropylene oxide) oligomers have shown to be non-bioaccumulable and non persistent [33]. They are usually produced by anionic ring opening oligomerization of hexafluoropropylene oxide (HFPO) (Scheme 5) [34, 35, 36, 37]. Various companies produce such perfluoropolyethers (PFPEs) Krytox® [38] or similar oligomers such as (CF2O)x(C2F4O)y or (CF2CF2CF2O)n.
|
Download:
|
|||
| Scheme. 5. Anionic ring-opening polymerization of HFPO. | ||||
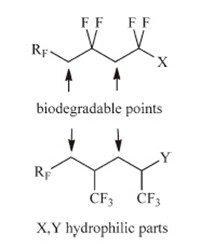
Potential degradability of surfactants can be possible if these compounds contain ‘‘weak points’’ such as a methylene or methine group (Fig. 1),which may undergo enzymatic or bio-degradation. So,this is considered when surfactants containing oligo(VDF) or oligo(TFP) chains as follows:
|
Download:
|
| Fig. 1. Surfactants containing ‘‘weak points’’. | |
Recently,Kappler and Lina [39] have prepared C2F5(VDF)n- CH2CO2H in four steps from the radical telomerization of VDF with C2F5I summarized as in Scheme 6. The overall yield fromC2F5I is 32%. The produced 3,3,5,5,7,7,8,8,8-nonafluorooctanoic acid contains the same number of carbon atoms of PFOA. The same strategy has also been successfully achieved fromC4F9I [40],interestingly,the surface tension of C4F9CH2CF2CH2CF2CH2COOH is similar to that of PFOA.

|
Download:
|
||||
| Scheme. 6. Preparation of an alternative to PFOA. | |||||
Though the telomerizations of TFP with various chlorinated or brominated chain transfer agents were achieved by Vasil’eva et al. [41, 42, 43, 44] or Zamyslov et al. [45, 46],few works have been reported on the radical telomerization of TFP with perfluoroalkyliodides [47, 48, 49]. These telomers have further been chemically modified into cationic surfactants according to the strategy shown in Scheme 7. Recently,ditert-butyperoxide was shown to be the most efficient initiator for the telomerization of TFP with dialkyl hydrogenophosphate. The reaction is displayed in Scheme 8 [50].
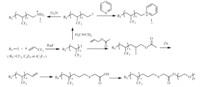
|
Download:
|
|||||
| Scheme. 7. Preparation of TFP-based cationic and non-ionic surfactants. | ||||||
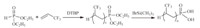
|
Download:
|
||||||
| Scheme. 8. Radical telomerization of TFP with diethyl hydrogenophonate followed by hydrolysis. | |||||||
Although the degradation of these surfactants containing VDF and TFP have not been achieved,these compounds are very interesting and simple reactions have been carried out in satisfactory yields. But,perfluoroalkyliodides are very expensive and are difficult to obtain. 2.3. Introducing branch
So far,basic research studies in this field revealed that straight chain fluorinated surfactants have the lowest surface tension at a relatively high concentration while the branched fluorinated surfactants show more efficient at a relatively low concentration. For example,Dmowski et al. [51] prepared CF3CF2CF2C(CF3)2CH2CH2 COONa and compared its surface activity with CF3(CF2)6COONa. The result showed that CF3CF2CF2C(CF3)2CH2CH2COONa has better surface ability than CF3(CF2)6COONa to reduce the surface tension of water at the same concentration. So,introducing branched structure in perfluorinated chain part is one of effective strategies for synthesis of non-bioaccumulable alternatives to PFOA [52].
Hexafluoropropylene oligomer is an important starting material, which can be used to synthesize many branched fluorinated surfactants and most reported methods are based on hexafluoropropylene trimer (HFPT). HFPT has three isomer structures (Fig. 2) while hexafluoropropylene dimer (HFPD) has two isomer structures as shown below. Both D-2 and T-2 have higher reactivity than their isomers. All of them are internal olefins containing multi-branches. Both the double bond and the fluorine atom directly attached to the carbon of the double bond are so active that they can easily react with a nucleophilic reagent in a polar solvent to form an intermediate containing reactive functional groups and thereby introducing the hydrophilic group into the surfactant.
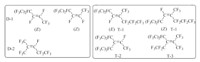
|
Download:
|
| Fig. 2. Isomer structures of HFPD and HFPT. | |
Nucleophilic substitution reaction on the fluorine atom. Reaction of HFPT and 4-hydroxybenzoic acid followed by react with SOCl2 lead to C9F17OC6H4COCl which can be used to prepare a variety of fluorinated surfactants (Scheme 9). A variety of non-ionic and cationic fluorinated surfactants can be prepared by reactions of HFPT with alcohols (Scheme 10). Reaction of HFPT with phenol lead to C9F17OC6H5,then active groups may be introduced on the phenyl ring of the compound C9F17OC6H5,thus various fluorinated surfactants can be made (Scheme 11). The Neos Company developed a series of surfactants through the above synthetic routes.
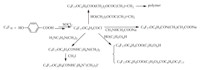
|
Download:
|
|||||||
| Scheme. 9. Products of reaction of HFPT and 4-hydroxybenzoic acid. | ||||||||
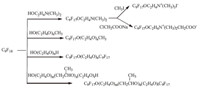
|
Download:
|
||||||||
| Scheme. 10. Products of reaction of HFPT and alcohols. | |||||||||
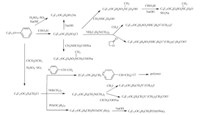
|
Download:
|
|||||||||
| Scheme. 11. Products of reaction of HFPT and phenol. | ||||||||||
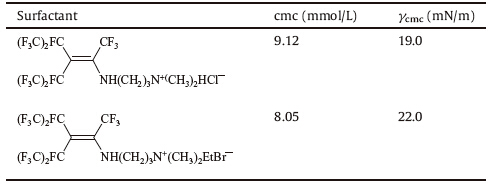
Liu et al. [53] investigated two cationic surfactants and their surface activities were studied. Results are shown in Table 1. All the values of their surface properties are lower than that of sodium perfluorooctanoate (about 24.7mN/mat the cmc 3.1×10-2 mol/L) [54].
| Table 1 Surface activities of the surfactants. |
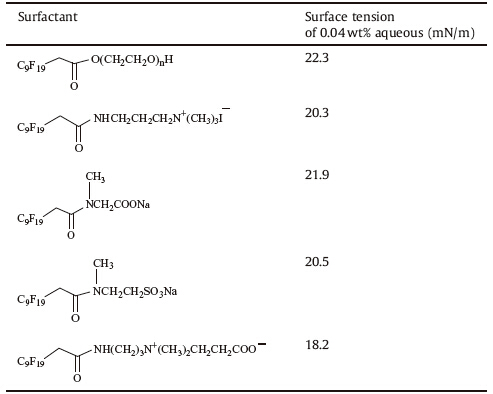
Nucleophilic addition reaction on the double bond. Mao et al. [55] synthesized different ionic fluorinated surfactants (Scheme 12),and their surface activities were determined (Table 2). The steps led to the products are as follows. Firstly,in the presence of fluoride ions,HFPT is changed to a carbanion intermediate followed by a nucleophilic substitution with methyl bromoacetate. Secondly,saponification of the ester followed by acidification lead to the carboxylic acid. Then,condensation of the acid with alcohol or amide leads to various surfactants.
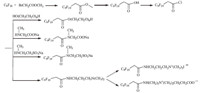
|
Download:
|
||||||||||
| Scheme. 12. Preparation of different ionic fluorinated surfactants. | |||||||||||
| Table 2 Surface tensions of the surfactants. |
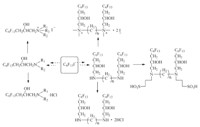
The methods for preparing branched fluorinated surfactants using hexafluoropropylene dimmer are extremely scanty [56, 57, 58, 59, 60, 61, 62]. Some Japanese patents reported the synthesis of surfactants derived from HFPD,such as JP3093744 and JP61069754 (Scheme 13). Other structural formulas reported are shown in Scheme 14. Their performances are no better than that of similar structural surfactants derived from HFPT.
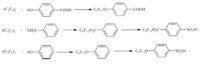
|
Download:
|
|||||||||||
| Scheme. 13. Surfactants reported in the patent JP3093744 and JP61069754. | ||||||||||||

|
Download:
|
||||||||||||
| Scheme. 14. Surfactants derived from HFPD. | |||||||||||||
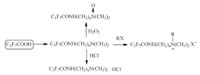
We also synthesized various surfactants (Scheme 15) derived from HFPD [63, 64]. All the steps were easy work-up,mild reaction conditions,low cost and high yields. Their surface activities were investigated. All the values of surface activities of the novel surfactants are lower than that of sodium perfluorooctanoate and the surfactants described in Scheme 13. Their low surface tension and cmc at room temperature make them potential surfactants.
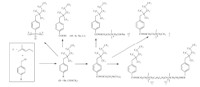
|
Download:
|
|||||||||||||
| Scheme. 15. Various surfactants derived from HFPD. | ||||||||||||||
Hexafluoropropylene is one of the most common fluorine chemical products,so it is suitable for industrial production of hexafluoropropene oligomers standing on the cost point. Hexafluoropropylene dimer contains only six carbons,so it is harmless to the environment. The methods for preparing branched fluorinated surfactants using hexafluoropropylene dimmer are extremely scanty. And the performances of some surfactants derived from HFPD are better than that of PFOA. So surfactants with excellent performance can be expected to be synthesized by molecular design. We believe that preparing branched fluorinated surfactants using HFPD will be the most environmental friendly and economical method.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21102167),the Science and Technology Commission of Shanghai Municipality (No. 12DZ1930902),Shanghai Green Chemical Engineering Technology Research Center and the Knowledge Innovation Program of the Chinese Academy of Sciences. We also thank Institute of Process Engineering,Chinese Academy of Sciences for helping us doing the surface tension measurements.
| [1] | A.X. Song, S.L. Dong, J.C. Hao, et al., Functions of fluorosurfactants 1: surface activities-improved and vesicle formation of the short-tailed chain sulfonate salt mixed with a fluorosurfactant, J. Fluor. Chem. 126 (2005) 1266-1273. |
| [2] | C.Q. Lu, J.H. Kim, D.D. DesMarteau, Synthesis of perfluoro-t-butyl trifluorovinyl ether and its copolymerization with TFE, J. Fluor. Chem. 131 (2010) 17-20. |
| [3] | T. Gramstad, R.N. Haszeldine, Perfluoroalkyl derivatives of sulphur. Part VII. Alkyl trifluoromethanesulphonates as alkylating agents, trifluoromethanesulphonic anhydride as a promoter for esterification, and some reactions of trifluoromethanesulphonic acid, J. Chem. Soc. (1957) 4069-4079. |
| [4] | M.P. Krafft, J.G. Riess, Perfluorocarbons: life sciences and biomedical uses dedicated to the memory of Professor Guy Ourisson, a true RENAISSANCE man, J. Polym. Sci. A: Polym. Chem. 45 (2007) 1185-1198. |
| [5] | R. Kaplánek, O. Paleta, I. Ferjentsiková, M. Kodíček, Novel perfluoroalkylated oligo(oxyethylene) methyl ethers with high hemocompatibility and excellent co-emulsifying properties for potential biomedical uses, J. Fluor. Chem. 130 (2009) 308-316. |
| [6] | L. Caillier, E.T. de Givenchy, R. Levy, et al., Polymerizable semi-fluorinated gemini surfactants designed for antimicrobial materials, J. Colloid Interface Sci. 332 (2009) 201-207. |
| [7] | J. Kovarova, Z. Svobodova, Perfluorinated compounds: occurrence and risk profile, Neuro Endocrinol. Lett. 29 (2008) 599-608. |
| [8] | D. Prescher, U. Gross, J. Wotzka, M. Tscheu-Schlü ter, W. Stark, Environmental behavior of fluoro surfactants. Part 2. Study on biochemical degradability, Acta Hydrochim. Hydrobiol. 13 (1985) 17-24. |
| [9] | B.D. Key, R.D. Howell, C.S. Criddle, Fluorinated organics in the biosphere, Environ. Sci. Technol. 31 (1997) 2445-2454. |
| [10] | B.D. Key, R.D. Howell, C.S. Criddle, Defluorination of organofluorine sulfur compounds by Pseudomonas Sp. Strain D2, Environ. Sci. Technol. 32 (1998) 2283-2287. |
| [11] | M. Houde, J.W. Martin, R.J. Letcher, K.R. Solomon, D.C.G. Muir, Biological monitoring of polyfluoroalkyl substances: a review, Environ. Sci. Technol. 40 (2006) 3463-3473. |
| [12] | C. Lu, Y.L. Shi, Z. Zhou, et al., Perfluorinated compounds in blood of textile workers and barbers, Chin. Chem. Lett. 25 (2014) 1145-1148. |
| [13] | K. Kannan, S. Corsolini, J. Falandysz, et al., Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries, Environ. Sci. Technol. 38 (2004) 4489-4495. |
| [14] | U.S. Environmental Protection Agency, http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html. |
| [15] | J.R. Parsons, M. Sáez, J. Dolfing, P. de Voogt, Biodegradation of Perfluorinated Compounds, Reviews of Environmental Contamination and Toxicology, vol. 196, Springer, New York, 2008, pp. 53-71. |
| [16] | K.A. Kennedy, G.W. Roberts, J.M. Desimone, Heterogeneous Polymerization of Fluoroolefins in Supercritical Carbon Dioxide, Polymer Particles, Advances in Polymer Science, vol. 175, Springer-Verlag, Berlin, 2005, pp. 329-346. |
| [17] | W.Y. Gu, D.D. Feng, X.L. Su, Progress of alternatives for PFOA, Organo-Fluor. Ind. 3 (2009) 28-30. |
| [18] | M.G. Wang, M.Y. Wang, Y.B. Tan, et al., Short fluorocarbon chain-based environment- friendly cationic fluorosurfactant and its preparation method, CN 102,814,144 A, 2012. |
| [19] | L. Wang, Synthesis and Properties of Perfluorobutylamide Fluorinated Surfactants, M.S. thesis, Shanxi University of Science and Technology, 2014. |
| [20] | G.D. Long, S. Xiao, D.M. Fu, J. Gulmira, X.S. Li, Synthesis and surface properties of perfluorobutyl cationic surfactant, Organo-Fluor. Ind. 3 (2012) 1-4. |
| [21] | G.D. Long, S. Xiao, J. Gulmira, et al., Synthesis and surface properties of N'- 3(dimethyl)-propyl-(N-perfluorobutyl sulfonyl-N-alkylsulfonyl)-amine oxide, Organo-Fluor. Ind. 4 (2012) 1-4. |
| [22] | B.Q. Yang, K. Chen, H. Xing, J.X. Xiao, Perfluorobutyl-based fluorinated surfactant with high surface activity, Acta Phys. Chim. Sin. 25 (2009) 2409-2412. |
| [23] | S. Xiao, Synthesis and Application on Substituting Fluorine Containing Surfactant for PFOS, M.S. thesis, Central China Normal University, 2013. |
| [24] | J. Gulimire, Synthesis and Application of Fluorinated Surfactants, M.S. thesis, Central China Normal University, 2014. |
| [25] | L. Peng, G.D. Long, S.L. Wang, et al., Synthesis and surface properties of N-3- (dimethylamino)-propyl perfluorohexylsulfonyl compounds, Fire Sci. Technol. 30 (2011) 937-939. |
| [26] | H.X. Shi, X.L. Qiu, H.K. Wu, L.J. Chen, J.P. Xiang, Synthesis and properties of ammonium salts of perfluorohexylsulfonamides, Fine Chem. 29 (2012) 438-442. |
| [27] | M.M. Yao, Y. Yang, M.Y. Xue, M. Lin, Synthesis and properties of novel fluorinated surfactant, N. Chem. Mater. 41 (2013) 73-75. |
| [28] | C. Zhang, Study on Synthesis and Properties of Fluorocarbon, M.S. thesis, Hubei University, 2013. |
| [29] | F.G. Drakesmith, D.A. Hughes, The electrochemical fluorination of octanoyl chloride, J. Appl. Electrochem. 9 (1979) 685-697. |
| [30] | R.N. Haszeldine, Synthesis of fluorocarbons, perfluoroalkyl iodides, bromides, and chlorides, and perfluoroalkyl Grignard reagents, Nature 167 (1951) 139-140. |
| [31] | H.J. Emeléus, R.N. Haszeldine, The reactions of fluorocarbon radicals. I. The reaction of iodotrifluoromethane with ethylene and tetrafluoroethylene, J. Chem. Soc. (1949) 2856-2861. |
| [32] | P.M. Murphy, C.S. Baldwin, R.C. Buck, Synthesis utilizing n-perfluoroalkyl iodides[RFI, CnF2n+1-I] 2000-2010, J. Fluor. Chem. 138 (2012) 3-23. |
| [33] | M.A. Guerra, K. Hintzer, M. Jurgens, et al., Fluorinated surfactants with low toxicity and/or little bioaccumulation, their manufacture and use, US 20,070,276,103 A1, 2007. |
| [34] | D.S. Slinn, S.W. Green, Fluorocarbon fluids for the use in the electronic industry, in: R.E. Banks (Ed.), Preparation, Properties and Industrial Applications of Organofluoride Compounds, vol. 2, Ellis Horwood, Chichester, 1982, pp. 45-82. |
| [35] | D. Sianesi, G. Marchionni, R.J. De Pasquale, Perfluoropolyethers from perfluoroolefin photooxidation, in: R.E. Banks, B.E. Smart, J.C. Tatlow (Eds.), Organofluorine Chemistry: Principles and Commercial Applications, Plenum Press, New York, 1994, pp. 431-460. |
| [36] | J. Scheirs, Perfluoropolyethers: synthesis, characterization and application, in: J. Scheirs (Ed.), Modern Fluoropolymers, Wiley, New York, 1997, pp. 435-486. |
| [37] | S.V. Kostjuk, E. Ortega, F. Ganachaud, B. Améduri, B. Boutevin, Anionic ringopening polymerization of hexafluoropropylene oxide using alkali metal fluorides as catalysts: a mechanistic study, Macromolecules 42 (2009) 612-619. |
| [38] | J.L. Howell, E.W. Perez, Perfluoropolyoxyalkylenes and perfluoropolyethers as lubricating oils and greases for magnetic recording devices, US 20,030,073,588 A1, 2003. |
| [39] | P. Kappler, M.J. Lina, Process for manufacture of fluoropolymers and surfactant for use in the process, FR 2,871,460 A1, 2005. |
| [40] | G. Boutevin, D. Tiffes, C. Loubat, B. Boutevin, B.Ameduri,Newfluorinated surfactants based on vinylidene fluoride telomers, J. Fluor. Chem. 134 (2012) 7-84. |
| [41] | T.T. Vasil’eva, V.A. Kochetkova, B.V. Nelyubin, et al., Radical telomerization of 3,3,3-trifluoro-1-propene with tetbromomethane. Individual chain transfer constants, Izv. Akad. Nauk SSSR, Ser. Khim. (1987) 808-811. |
| [42] | T.T. Vasil’eva, V.A. Kochetkova, V.I. Dostovalova, et al., Reaction of 3,3,3-trifluoro- 1-propene with bromoform and methylene bromide, Izv. Akad. Nauk SSSR, Ser. Khim. (1989) 2558-2562. |
| [43] | T.T. Vasil’eva, I.A. Fokina, S.V. Vitt, et al., Radical telomerization of 3,3,3-trifluoropropene- 1 with CCl4, Izv. Akad. Nauk SSSR, Ser. Khim. (1990) 1807-1811. |
| [44] | T.T. Vasil’eva, I.A. Fokina, S.V. Vitt, Reaction of benzyl chloride with propylene and trifluoropropene under metal complex initiation conditions, Izv. Akad. Nauk SSSR, Ser. Khim. (1991) 1384-1388. |
| [45] | R.A. Zamyslov, A.G. Shostenko, I.V. Dobrov, V.E. Myshkin, Radical telomerization of 3,3,3-trifluoro-1-propene with 2-propanol, Zh. Org. Khim. 16 (1980) 897-901. |
| [46] | R.A. Zamyslov, Reactivity of C1-C4 aliphatic alcohols in radical telomerization with 3,3,3-trifluoropropylene, Zh. Vses. Khim. Obsh. 31 (1986) 589-591. |
| [47] | R.N. Haszeldine, Reactions of fluorocarbon radicals. V. Alternative synthesis for (trifluoromethyl)acetylene (3,3,3-trifluoropropyne) and the influence of polyfluoro groups on adjacent hydrogen and halogen atoms, J. Chem. Soc. (1951) 2495-2504. |
| [48] | R.N. Haszeldine, The addition of free radicals to unsaturated systems. I. The direction of radical addition to 3,3,3-trifluoropropene, J. Chem. Soc. (1952) 2504-2513. |
| [49] | G.K. Kostov, B. Ameduri, S.M. Brandstadter, Telomerization of 3,3,3-trifluoroprop- 1-ene and functionalization of its telomers, Collect. Czechoslov. Chem. Commun. 73 (2008) 1747-1763. |
| [50] | G. Kostov, B. Améduri, S.M. Brandstadter, Radical telomerization of 3,3,3-trifluoropropene with diethyl hydrogen phosphonate: characterization of the first telomeric adducts and assessment of the transfer constants, J. Fluor. Chem. 128 (2007) 910-918. |
| [51] | W. Dmowski, H. Plenkiewicz, K. Piasecka-Maciejewska, Synthetic utility of 3- (perfluoro-1,1-dimethylbutyl)-1-propene. Part III. Synthesis and properties of (perfluoro-1,1-dimethylbutyl) acetic and propionic acids and their salts, J. Fluor. Chem. 48 (1990) 77-84. |
| [52] | G. Kostov, F. Boschet, B. Ameduri, Original fluorinated surfactants potentially nonbioaccumulable, J. Fluor. Chem. 130 (2009) 1192-1199. |
| [53] | Z.M. Liu, C.M. Tan, J.F. Wu, et al., Synthesis of fluoro-tertiary and quaternary ammonium cationic surfactants, Dyest. Color. 43 (2006) 31-33. |
| [54] | Q.S. Zhang, Z.Y. Luo, D.P. Curran, Separation of "light fluorous" reagents and catalysts by fluorous solid-phase extraction: synthesis and study of a family of triarylphosphines bearing linear and branched fluorous tags, J. Org. Chem. 65 (2000) 8866-8873. |
| [55] | S.Q. Mao, M.F. Tang, C. Gu, Synthesis, surface activities and oil collecting performance of different ionic fluorinated surfactants derived from hexafluoropropylene trimer, Organo-Fluor. Ind. 1 (1996) 1-5. |
| [56] | I. Ikeda, M. Tsuji, M. Okahara, Fluoro surfactants, tenside, surfactants, Detergents 24 (1987) 272-274. |
| [57] | H. Tomota, N. Nakayama, Preparation of fluorine-containing aromatic carboxylic acids as intermediates for surfactants and polymers, JP 03,093,744 A, 1991. |
| [58] | Z.M. Liu, J.F. Wu, L.M. Tan, et al., Synthesis and properties of cationic surfactant from hexafluoropropene dimer, Fine Chem. 22 (2005) 53-55. |
| [59] | B.Q. Yang, L. Wang, J. Yang, J. Liu, Synthesis and properties of fluorinated surfactants based on hexafuoropropene dimmer, Chem. Res. Appl. 25 (2013) 1523-1527. |
| [60] | T.C. Zheng, Y.J. Zhao, Z.G. Lei, Q. Zhou, S.H. Wang, Research progress on performance and synthesis of surfactants based on hexafluoroprpene oligomers, Chem. Prod. Technol. 19 (2012) 6-11. |
| [61] | H. Qi, W.G. Xu, W.J. Zhao, et al., Hexafluoropropylene dimer and its applications, Organo-Fluor. Ind. 4 (2012) 18-22. |
| [62] | J.H. Xu, Z.J. Chen, W.B. Zhang, Hexafluoropropylene dimers and derivatives thereof, Organo-Fluor. Ind. 3 (2007) 29-32. |
| [63] | M. Sha, R.M. Pan, L.W. Zhan, P. Xing, B. Jiang, Synthesis and surface activity study of a novel branched fluorinated anion surfactant with CF3CF2CF2C(CF3)2 group, Chin. J. Chem. 32 (2014) 995-998. |
| [64] | M. Sha, R.M. Pan, P. Xing, B. Jiang, Synthesis and surface activity study of branched fluorinated cationic (FCS), gemini (FGS) and amphoteric (FAS) surfactants with CF3CF2CF2C(CF3)2 group, J. Fluor. Chem. 169 (2015) 61-65. |