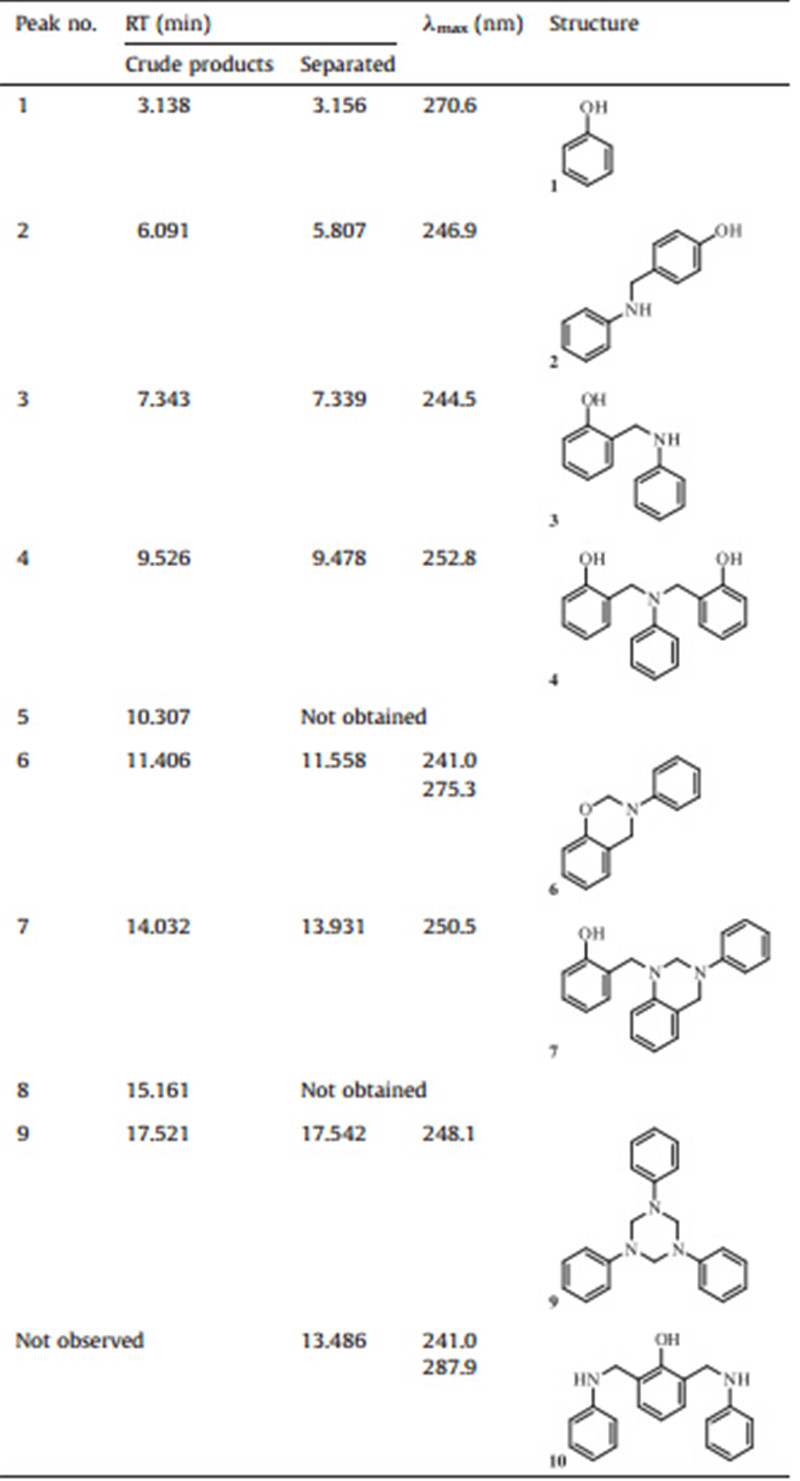
Polybenzoxazines,obtained via thermal polymerization of corresponding benzoxazine monomers,have received widespread research interest because of their excellent performance [1, 2, 3, 4, 5, 6, 7]. The synthesis of benzoxazine monomer was first reported by Holly and Cope [8],in which it can be obtained by a variety of synthetic routes [9, 10, 11, 12]. The most universal route is using phenols,primary amines,and formaldehyde as starting materials [3, 13, 14, 15, 16, 17, 18]. For this route,Burke proposed the possible reaction path:N,N-dihydroxymethyl amine (I) is initially generated (Scheme 1,step 1) and then converted into 2-(N-hydroxymethyl-N-substituted amino)methylphenol (N-hydroxymethyl Mannich base) (II) (Scheme 1,step 2). Benzoxazine (III) is then formedviathe dehydration reaction ofNmethylol and phenol hydroxyl group (Scheme 1,step 3) [1, 2, 13]. Moreover,many studies about the effects of different reactant structures [13, 16, 19, 20, 21, 22],reactant ratios [23, 24],reaction temperatures [15],solvent effect [25],and reaction duration [22] have also been reported; results showed that these conditions can influence benzoxazine yield and generate various byproducts.
|
Download:
|
| Scheme 1.Reported reactions in benzoxazine synthesis from phenol,primary amine,and formaldehyde [1] | |
Many studies have focused on synthesizing novel benzoxazine monomers [26, 27, 28]. However,a number of factors,including the formation of byproducts,remain unresolved,leading to the low yield and poor purity of benzoxazine monomers,which may complicate the purification process or influence the properties of polybenzoxazines. These problems will further limit the development of benzoxazine. Nonetheless,to the best of our knowledge, the compositions and chemical structures of the synthetic products have not been discussed in detail. The chemical composition of the synthetic product is only a general description of the product,dimer,and oligomer,and these indistinct conclusions are insufficient to control the synthesis reaction from the perspective of the reaction mechanism. Moreover,characterizing the composition and chemical structure in a reaction mixture is difficult with the use of spectroscopic techniques. Further studies on the products of benzoxazine synthesis are needed.
This work aims to study the products from the reactions among phenol,aniline,and formaldehyde. Elucidation of the compositions and chemical structures of the crude products can provide information in understanding the reaction paths in benzoxazine synthesis. Thus,we focus on separating the compounds from the crude products by using high-performance liquid chromatography (HPLC),column chromatography (CC),and preparative HPLC. The separated compounds are characterized by nuclear magnetic resonance (NMR) and mass spectrometry (MS). Reaction paths of the synthesis from phenol,aniline,and formaldehyde are proposed based on the results.N-Hydroxymethyl aniline (HMA) derived from the reaction between formaldehyde and aniline is probably the key intermediate during the reaction. The results are beneficial for further fundamental and systematic studies on the mechanism of the benzoxazine synthesis. 2. Experiment
2.1. Materials and measurements
Phenol and aniline (≥99%,ACS) were obtained from Aladdin Chemistry Co.,Ltd. Paraformaldehyde (≥98%) was acquired from Ercros Industrial S.A. Spain. Dioxane (≥99%),toluene (≥99%), ethanol (≥99%),and sodium sulfite (≥97%) were purchased from the Chengdu Kelong Chemical Reagents Corp. (China). All of the reagents were used as received. Silica gel (200-300 mesh) for column chromatography (CC) was purchased from Qingdao Haiyang Chemical Co.,Ltd. (China).
1HNMR, 13CNMR and 2D NMR spectra were performed on a Bruker AV II-600 NMR,in which deuterated dimethyl sulfoxide (DMSO-d6) was used as solvent and tetramethylsilane as internal standard.
Analytical HPLC was performed on a Waters 2695 Separations Module equipped with a Waters 2996 phodediode array detector and Empower workstation software (Waters,Milford,MA,USA). The CC was a SunFire C18 (150 mm×4.6 mm,I.D. 5mm) (Waters, Milford,MA,USA). The gradient program was as follows: step 1, 45-95% acetonitrile over 25 min; step 2,95% acetonitrile over 5 min; step 3,45% acetonitrile over 5 min. The mobile phase was a mixture of acetonitrile and water.
Preparative HPLC separations were performed on a PACK-NSEPTM dynamic axial chromatographic column: LC50.340.VE100 PS TH (I.D. 50 mm,length 340 mm,NovaSep,Pompey,France). Packing material was ODS (S-10mm,YMC Co.,Ltd.,Japan). The column yielded a bed volume of 625 mL and void volume of 212 mL. The preparative HPLC system was equipped with HPG500 Pump (Sunyear Scientific Inc.,Shanghai,China) and was monitored by a Smartline UV 2500 detector (Knauer,Berlin,Germany). Calesep workstation version 2.22 was used as the workstation (Sunyear Scientific Inc.,Shanghai,China).
All of the mass spectra were acquired using a Micromass Q-TOF micro mass spectrometer (Waters Corp.,Milford,MA,USA) equipped with electrospray ionization source. All of the operations, as well as data acquisition and analyses,were controlled using Masslynx V4.1 software (Waters Corp.,Milford,MA,USA). 2.2. Preparation of aqueous formaldehyde solution
The aqueous formaldehyde solution was prepared as follows: Approximately 70 g of water was adjusted to pH 8 using 4% NaOH solution. Paraformaldehyde (30 g) was added,and the mixture was stirred at 70℃ for 1 h to form a transparent solution with pH 5-6. The concentration of formaldehyde was confirmed by titration with sodium sulfite. 2.3. Synthesis of 3,4-dihydro-2H-3-phenyl-1,3-benzoxazine
Stoichiometric amounts of aniline (0.2 mol,18.6 g),phenol (0.2 mol,18.8 g) and aqueous formaldehyde solution (0.4 mol, 32.5 g) were dissolved in dioxane (50 mL) in a 150 mL threenecked flask. The mixture was stirred and refluxed at 80℃ for 5 h. The crude products were dried with anhydrous sodium sulfate,and then the solvent was removed by rotary evaporation.
The crude products were separated firstly using gradient solution column chromatography. Then the separative products were further separated and purified by preparative HPLC. The analytical data of the compounds separated from the crude products were as follow:
·4-((Phenylamino)methyl)phenol (2): 1HNMR (600 MHz,DMSOd6):δ9.23 (s,1H),7.14 (d,2H,J= 8.4 Hz),7.01 (d,2H,J= 8.4 Hz), 6.69 (t,2H,J= 4.3 Hz),6.56 (d,2H,J= 7.9 Hz),6.48 (d,1H, J= 7.1 Hz),6.01 (s,1H),4.11 (d,2H,J= 5.9 Hz). 13CNMR (151 MHz,DMSO-d6):δ 156.56,149.24,130.63,129.20, 128.91,116.02,115.47,112.71,46.55. HRMS (ESI) m/z 200.1073 [(MH)+ ; calcd. for C13H14NO: 200.1075].
·2-((Phenylamino)methyl)phenol (3): 1HNMR (600 MHz,DMSOd6):δ9.49 (s,1H),7.17 (d,1H,J= 7.4 Hz),7.03 (t,3H,J= 7.8 Hz), 6.81 (d,1H,J= 8.0 Hz),6.72 (t,1H,J= 7.4 Hz),6.56 (d,2H, J= 8.0 Hz),6.49 (t,1H,J= 7.2 Hz),5.97 (t,1H,J= 5.9 Hz),4.18 (d, 2H,J= 5.9 Hz). 13CNMR (151 MHz,DMSO-d6):δ155.47,149.34, 129.23,128.69,127.88,126.27,119.22,116.07,115.36,112.67, 41.88.
·2,2' -((Phenylimino)bis(methylene))bisphenol (4): 1HNMR (600 MHz,DMSO-d6):δ9.57 (s,1H),7.06 (q,2H,J= 8.0 Hz),6.99 (d,1H, J= 7.4 Hz),6.84 (d,1H,J= 7.9 Hz),6.72 (t,1H,J= 7.4 Hz),6.57 (d, 1H,J= 8.5 Hz),6.54 (d,1H,J= 7.2 Hz),4.54 (s,2H). 13CNMR (151 MHz,DMSO-d6):δ155.51,148.99,129.38,127.92,127.39, 124.65,119.30,115.97,115.42,112.07,49.80. HRMS (ESI)m/z 306.1497 [(MH)+ ; calcd. for C20H20NO2: 306.1494].
·3,4-Dihydro-2H-3-phenyl-1,3-benzoxazine (6): 1HNMR (600 MHz,DMSO-d6):δ7.23 (t,2H,J= 7.9 Hz),7.13 (d,2H,J= 8.3 Hz), 7.10 (s,1H),7.08 (t,1H,J= 7.8 Hz),6.85 (t,2H,J= 7.5 Hz),6.72 (d, 1H,J= 8.1 Hz),5.44 (s,2H),4.65 (s,2H). 13CNMR (151 MHz, DMSO-d6):δ 154.43,148.27,129.56,128.10,127.64,121.78, 120.93,120.89,117.80,116.68,79.11,49.36.
·2-((3-Phenyl-3,4-dihydroquinazolin-1(2H)-yl)methyl)phenol (7): 1HNMR (600 MHz,DMSO-d6):δ9.59 (s,1H),7.19 (t,2H, J= 7.9 Hz),7.07-6.99 (m,4H),6.97 (d,1H,J= 7.2 Hz),6.91 (t,1H, J= 7.4 Hz),6.84 (d,1H,J= 7.9 Hz),6.78 (t,1H,J= 7.2 Hz),6.64 (t, 1H,J= 7.4 Hz),6.56 (t,1H,J= 7.3 Hz),6.42 (d,1H,J= 8.2 Hz),4.85 (s,2H),4.58 (s,2H),4.39 (s,2H,). 13CNMR (151 MHz,DMSO-d6):δ 155.40,149.27,145.20,129.41,128.23,128.05,127.75,127.04, 124.70,120.58,119.67,119.20,116.94,116.87,115.41,112.04, 66.88,50.99,48.46. HRMS (ESI)m/z317.1650 [(MH)+ ; calcd. for C21H21N2O: 317.1654].
·1,3,5-Triphenyl-1,3,5-triazinane (9): 1HNMR (600 MHz,DMSOd6):δ7.23-7.15 (m,1H),7.06 (d,1H,J= 7.9 Hz),6.78 (t,1H, J= 7.2 Hz),4.90 (s,1H). 13CNMR (151 MHz,DMSO-d6):δ148.25, 128.90,119.86,116.80,66.95.
·2,6-Bis((phenylamino) methyl) phenol (10): 1HNMR (600 MHz, DMSO-d6):δ8.84 (s,1H),7.10 (d,2H,J= 7.5 Hz),7.05 (t,4H, J= 7.8 Hz),6.74 (t,1H,J= 7.5 Hz),6.60 (d,4H,J= 7.9 Hz),6.53 (t,2H,J= 7.2 Hz),6.01 (t,2H,J= 5.2 Hz),4.27 (d,4H,J= 5.1 Hz). 13CNMR (151 MHz,DMSO-d6):δ153.16,149.22,129.28,127.09, 126.95,119.71,116.49,113.00,65.39,42.82. HRMS (ESI)m/z 305.1652 [(MH)+ ; calcd. for C20H21N2O: 305.1654]. 3. Results and discussion 3.1. Composition analysis of the crude products
The reaction involving phenol,aniline,and formaldehyde at a molar ratio of 1:1:2 occurred in the presence of dioxane. After this reaction,the crude products were characterized by HPLC and the results are presented in Fig. 1. Nine peaks (peaks 1-9) are found, which probably suggest that the crude products contain nine components. In order to study these components,CC and preparative HPLC were applied to separate and purify the crude products. Seven compounds were obtained,and their retention times (RT) and UV absorption were assessed by HPLC. These compounds corresponding to the peaks of 1,2,3,4,6,7 and 9 (Fig. 1) were obtained. The chemical structures of these compounds were elucidated by NMR and MS (see Supporting information) and summarized in Table 1.

|
Download:
|
| Fig. 1. The HPLC result of the crude products from the reaction among phenol, aniline and formaldehyde. | |
| Table 1 Identification of compounds in the crude product from the reaction among aniline, phenol and formaldehyde. |
As shown in Table 1,almost no aniline was detected,whereas a small amount of phenol (1) remained after reaction,and the most abundant compound in these crude products was benzoxazine (6). Three phenylaminomethyl moiety-containing compounds (compounds2,3and10) were obtained. The difference between the isomers (2and3) is the substituted positions of phenylaminomethyl on phenolic rings. For compound 10,two ortho positions of the hydroxyl group were substituted by phenylaminomethyl. In addition,the tertiary amine moieties were observed in compound4,7,and 9. In the separation results, the compounds corresponding to peaks 5 and 8 in Fig. 1 were not obtained. Preparative HPLC was applied to separate the two compounds,which were separated from the crude products and monitored by UV-detector. However,when we used HPLC to confirm the collected solution,the compounds corresponding to peaks 5 and 8 were observed,and several new compounds were also detected. The phenomena showed these two compounds corresponding to peaks 5 and 8 were unstable in the eluent,and can partially convert into some new compounds easily. Therefore, resulting in no pure compounds corresponding to peaks 5 and 8 were gained. Moreover,a compound with RT of 13.486 min was separated from the crude products,but almost no corresponding peak was observed in Fig. 1. This is probably because the compound accumulated during the repeated separation processes to a level that met the minimum requirement of separation experiment,even though this compound was only a small fraction of the crude products. 3.2. Reaction path inferred from the composition of synthetic products
As mentioned above,this work aims to investigate the reaction path of benzoxazine synthesis by analyzing the composition of the final products. Previous studies claimed that the benzoxazine synthesis is initiated by the reaction between primary amine and formaldehyde to form the highly reactive intermediate N,Ndihydroxymethylamine,which subsequently reacted with other reactants or intermediates (Scheme 1,step 1) [13],resulting in tertiary amine compounds. According to this hypothesis,no secondary amine compounds should be formed during this reaction. However,three secondary amine compounds 2,3 and 10were found in the final products. Among all these compounds, the generation of compound 3 can be explained by the hydrolysis reaction of benzoxazine monomer. Other compounds such as compounds 2and 10are very difficult to obtain if the first step of the reaction is indeed between formaldehyde and primary amine to generateN,N-dihydroxymethlyamine. Therefore,N,N-dihydroxymethlyamine is not likely the intermediate during benzoxazine synthesis. A more reasonable intermediate probably isN-hydroxymethyl aniline (HMA),which could be further transformed to compounds2,3and10,although no existence of HMA could be confirmed yet [29, 30]. In addition,in a previous report about benzoxazine synthesis [10],the active intermediate of compound9 could also be generated from the self-reaction of HMA,which further imply that HMA is possibly the key intermediate. The probable path of the reaction may be as following: formaldehyde initially reacts with primary amine to generate HMA,which subsequently reacts with other reactants and intermediates to give the final products.
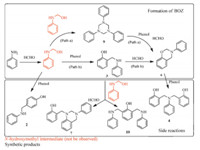
To illustrate the reaction path during benzoxazine synthesis, the reactions generated benzoxazine are denoted as the main reaction,whereas the others are considered side reactions. Based on the compositions and chemical structures of the synthetic products,in the case of the main reaction,the favored path of benzoxazine that was synthesized from phenol,aniline,and formaldehyde is proposed (Scheme 2). The first step of reaction is the formation of active intermediate HMA,which is derived from the reaction between formaldehyde and primary amine. One HMA molecule then reacts with the other HMA molecule to generate compound9,which can be converted into benzoxazine by reacting with paraformaldehyde and phenol [10] (Scheme 2,path a). However, if HMA attacks at the orthoposition of phenol,then it will form compound 3,which can also immediately transform into benzoxazineviareaction with formaldehyde (Scheme 2,path b) [31].
For the side reactions,when one HMA attack at the para position of phenol,compound 2i s formed. Compound 10can be generated if HMA attacks at theorthoposition of phenol ring of compound 3. However,if compound 3 reacts with HMA and formaldehyde,compound 7,which has a -N-CH2-N- structure,is generated. Given that noN-hydroxymethyl Mannich base was found in final products,compound 4 probably stems from the reaction between benzoxazine and phenol as previously reported [13].
|
Download:
|
| Scheme 2.Possible reaction paths for the benzoxazine synthesis from phenol, aniline and formaldehyde. | |
Results of the reaction path indicate that HMA derived from the reaction of aniline and formaldehyde is the key intermediate during the synthesis of benzoxazine. Although HMA was not observed in final products because of its high activity,it is nonetheless acceptable given that the path can illustrate the experiment very well. The reaction path of benzoxazine synthesis is controlled by competing reactions of HMA with different reactants and intermediates,and these competing reactions determine the type and amount of the final products. 4. Conclusion
In this work,the synthetic products of the reaction among phenol,aniline,and formaldehyde were studied in detail. Seven compounds were obtained and characterized. A possible reaction path of benzoxazine synthesis from phenol,aniline and formaldehyde was proposed based on the results. HMA,generated firstly from the reaction of formaldehyde and primary amine,is the key intermediate. HMA can attack at the orthoposition of phenol to generate compound 3,which can immediately react with formaldehyde to form benzoxazine. HMA can also react with formaldehyde and aniline to form compound 9,which can transform into benzoxazine through its reaction with phenol and formaldehyde. However,when HMA reacts with other intermediates and reactants,side reactions occur to form byproducts such as compound 2. The results of this study will help researchers to understand the synthesis of benzoxazine,as well as the design and development of novel benzoxazines.
Acknowledgment This work was supported by the National Natural Science Foundation of China (No. 21174093). Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.12.005.| [1] | H. Ishida, T. Agag, Handbook of Benzoxazine Resins, Elsevier, New York, 2011. |
| [2] | N. Ghosh, B. Kiskan, Y. Yagci, Polybenzoxazines-new high performance thermosetting resins: synthesis and properties, Prog. Polym. Sci. 32 (2007) 1344-1391. |
| [3] | X. Ning, H. Ishida, Phenolic materials via ring-opening polymerization: synthesis and characterization of bisphenol-A based benzoxazines and their polymers, J. Polym. Sci. Part A: Polym. Chem. 32 (1994) 1121-1129. |
| [4] | S.K. Kim, S.W. Choi, W.S. Jeon, et al., Cross-linked benzoxazine-benzimidazole copolymer electrolyte membranes for fuel cells at elevated temperature, Macromolecules 45 (2012) 1438-1446. |
| [5] | R. Kudoh, A. Sudo, T. Endo, A highly reactive benzoxazine monomer, 1-(2-hydroxyethyl)-1,3-benzoxazine: activation of benzoxazine by neighboring group participation of hydroxyl group, Macromolecules 43 (2010) 1185-1187. |
| [6] | S.F. Li, Synthesis of benzoxazine-based phenolic resin containing furan groups, Chin. Chem. Lett. 21 (2010) 868-871. |
| [7] | P. Yang, Y. Gu, Synthesis of a novel benzoxazine containing benzoxazole structure, Chin. Chem. Lett. 21 (2010) 558-562. |
| [8] | F.W. Holly, A.C. Cope, Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydroxybenzylamine, J. Am. Chem. Soc. 66 (1944) 1875-1879. |
| [9] | M.C. Aversa, P. Giannetto, C. Caristi, A. Ferlazzo, Behaviour of an N-(o-hydroxybenzyl)-b-amino-acid in the presence of dehydrating agents. Synthesis of a 3,4-dihydro-2H-1,3-benzoxazine, J. Chem. Soc. Chem. Commun. (1982) 469-470. |
| [10] | Z. Brunovska, J.P. Liu, H. Ishida, 1,3,5-Triphenylhexahydro-1,3,5-triazine-active intermediate and precursor in the novel synthesis of benzoxazine monomers and oligomers, Macromol. Chem. Phys. 200 (1999) 1745-1752. |
| [11] | Y.L. Liu, J.M. Yu, C.I. Chou, Preparation and properties of novel benzoxazine and polybenzoxazine with maleimide groups, J. Polym. Sci. Part A: Polym. Chem. 42 (2004) 5954-5963. |
| [12] | C.H. Lin, S.L. Chang, C.W. Hsieh, H.H. Lee, Aromatic diamine-based benzoxazines and their high performance thermosets, Polymer 49 (2008) 1220-1229. |
| [13] | W.J. Burke, 3,4-dihydro-1,3,2H-benzoxazines. Reaction of p-substituted phenols with N,N-dimethylolamines, J. Am. Chem. Soc. 71 (1949) 609-612. |
| [14] | W.J. Burke, C.W. Stephens, Monomeric products from the condensation of phenol with formaldehyde and primary amines, J. Am. Chem. Soc. 74 (1952) 1518-1520. |
| [15] | W.J. Burke, M.J. Kolbezen, C.W. Stephens, Condensation of naphthols with formaldehyde and primary amines, J. Am. Chem. Soc. 74 (1952) 3601-3605. |
| [16] | W.J. Burke, K. Murdock, G. Ec, Condensation of hydroxyaromatic compounds with formaldehyde and primary aromatic amines, J. Am. Chem. Soc. 76 (1954) 1677-1679. |
| [17] | H. Ishida, Process for preparation of benzoxazine compounds in solventless systems, US 5543516, 1996. |
| [18] | W.J. Burke, C. Weatherbee, H. Lau, G.V. Lear, G. Goken, Mono-1,3-benzoxazines from hydroquinone, J. Org. Chem. 28 (1963) 1098-1100. |
| [19] | W.J. Burke, E.L.M. Glennie, C. Weatherbee, Condensation of halophenols with formaldehyde and primary amines, J. Org. Chem. 29 (1964) 909-912. |
| [20] | X.Y. Wang, F. Chen, Y. Gu, Influence of electronic effects from bridging groups on synthetic reaction and thermally activated polymerization of bisphenol-based benzoxazines, J. Polym. Sci. Part A: Polym. Chem. 49 (2011) 1443-1452. |
| [21] | W.J. Burke, C. Weatherbee, 3,4-dihydro-1,3,2H-benzoxazines. Reaction of polyhydroxybenzenes with N-methylolamines, J. Am. Chem. Soc. 72 (1950) 4691-4694. |
| [22] | W.J. Burke, C.R. Hammer, C. Weatherbee, Bis-m-oxazines from hydroquinone, J. Org. Chem. 26 (1961) 4403-4407. |
| [23] | W.J. Burke, R.P. Smith, C. Weatherbee, N,N-bis-(hydroxybenzyl)-amines: synthesis from phenols, formaldehyde and primary amines, J. Am. Chem. Soc. 74 (1952) 602-605. |
| [24] | S. Chirachanchai, A. Laobuthee, S. Phongtamrug, Self termination of ring opening reaction of p-substituted phenol-based benzoxazines: an obstructive effect via intramolecular hydrogen bond, J. Heterocycl. Chem. 46 (2009) 714-721. |
| [25] | A. Laobuthee, S. Chirachanchai, H. Ishida, K. Tashiro, Asymmetric mono-oxazine: an inevitable product from mannich reaction of benzoxazine dimers, J. Am. Chem. Soc. 123 (2001) 9947-9955. |
| [26] | J. Liu, X. Lu, Z. Xin, C.L. Zhou, Synthesis and surface properties of low surface free energy silane-functional polybenzoxazine films, Langmuir 29 (2012) 411-416. |
| [27] | Y.R. Cheng, J. Yang, Y.X. Jin, D.Y. Deng, F. Xiao, Synthesis and properties of highly cross-linked thermosetting resins of benzocyclobutene-functionalized benzoxazine, Macromolecules 45 (2012) 4085-4091. |
| [28] | H.C. Chang, C.H. Lin, Y.W. Tian, Y.R. Feng, L.H. Chan, Synthesis of 9,9-bis(4-aminophenyl) fluorine-based benzoxazine and properties of its high-performance thermoset, J. Polym. Sci. Part A: Polym. Chem. 50 (2012) 2201-2210. |
| [29] | M.A. Sprung, A summary of the reactions of aldehydes with amines, Chem. Rev. 26 (1940) 297-338. |
| [30] | W.R. Abrams, R.G. Kallen, Equilibriums and kinetics of N-hydroxymethylamine formation from aromatic exocyclic amines and formaldehyde. Effects of nucleophilicity and catalyst strength upon mechanisms of catalysis of carbinolamine formation, J. Am. Chem. Soc. 98 (1976) 7777-7789. |
| [31] | Y.Y. Deng, Q. Zhang, H.C. Zhang, et al., Kinetics of 3,4-dihydro-2H-3-phenyl-1, 3-benzoxazine synthesis from Mannich base and formaldehyde, Ind. Eng. Chem. Res. 53 (2014) 1933-1939. |