b State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China
Derivatives from 2,2' :6' ,2'' -terpyridine (TPy) have attracted much attention because they can act as functional templates or building blocks in the fields of supramolecular and coordination chemistry as well as material science [1, 2]. They are not only important light-harvesting units,but also are of strong coordinative ability to a variety of transition metal ions to form diverse metal-complexes and metallo-supramolecular architectures. The as-prepared metal-organic complexes have interesting redox and photophysical properties as well as catalytic ability to many organic reactions,resulting in potential applications in the fields of light-to-electricity conversion,organic light-emitting diodes, sensors and non-linear optical devices [3, 4]. They can also act as building blocks to construct well-defined two- or three-dimensional multilayers on the solid surfaces for the development of nanoscale materials and devices [5].
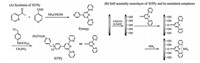
Here,we reported a newly synthesized silylated TPy derivative (SiTPy) based on an addition reaction of 4-(chloromethyl)phenyltrimethoxysilane with a multidentate ligand of 4' -(4-pyridyl)-2,2' :6' ,2'' -terpyridine (Pyterpy) that contains another pyridyl coordinative site at the 4' -position of TPy [6]. After the addition reaction,the SiTPy as-prepared contains a substituent of trimethoxysilane at the 4' -position of TPy,leading to a possibility to form organic-inorganic hybrid nanomaterials or composites [7], as well as a possibility to fabricate self-assembled monolayers (SAMs) on the hydrophilic solid supports [8]. In the present letter, the luminescent properties of SiTPy and its metalated complexes were investigated in the solutions and SAMs. Our results revealed that the present silylated-TPy compound gave off a luminescent emission at about 456 nm,which shifted to 452 nm for its metalated complexes with the transition metal ions of Zn(II) and Fe(II). Metal-SiTPy SAMs could be obtained by immersing the SiTPy SAMs in the solutions of metal ions. The energy absorbed by the ligand of SiTPy can be transferred to the lanthanide ions (Tb3+ and Eu3+ ) to give off the typical emissions of the lanthanide complexes. It was revealed that,different from that in the Zn2+ / Fe2+ -SiTPy complexes,the ligand fluorescence emission shifted to about 363 nm in the Ln3+ -SiTPy complexes,which may be attributed to the reason that the energy in the lower excited level (about 456 nm) could be more effectively transferred to the excited state of the lanthanide ions. 2. Experimental
2.1. Materials
2-Acetylpyridine,pyridine-4-carbaldehyde,4-(chloromethyl)-phenyltrimethoxysilane,zinc trifluoromethanesulfonate,europium trifluoromethanesulfonate,terbium trifluoromethanesulfonate,and iron tetrafluoroborate hexahydrate were purchased from Acros Organics. All chemicals were used as received without further purification. 2.2. Synthesis of silylated-terpyridine
The ligand Pyterpy (Fig. 1) was synthesized according to the literature method [9, 10]. Briefly,a mixture of 2-acetylpyridine, pyridine-4-carbaldehyde potassium hydroxide and ammonia water was stirred in the water-ethanol solution at room temperature for several hours. A purple solid was obtained and well-washed by cold ethanol and driedin vacuo. Finally,the crude product was recrystallized from methanol/chloroform (1:1) to produce small white crystals.
Silylated terpyridine derivative of SiTPy was synthesized by refluxing a mixture of Pyterpy with excess 4-(chloromethyl)phenyltrimethoxysilane in the nitromethane solution for 8 h. After addition of excess diethyl ether in the reaction system,the yellow gray solid powders were obtained,which were filtered and well washed by diethyl ether. To purify,the solid powders were dissolved in the nitromethane,precipitated again after addition of diethyl ether and finally driedin vacuo. 1HNMR (CD3OD):δ9.34 (2H),8.88 (2H),8.77 (4H),8.68 (2H),8.06 (2H),7.65 (2H),7.57 (2H), 7.51 (2H),5.92 (2H).
|
Download:
|
| Fig. 1. Schematic representation for (A) synthesis of the silylated-TPy derivative and (B) self-assembled monolayers of SiTPy and its metalated complexes. | |
The SAMs of SiTPy were prepared by immersing a freshly cleaned hydrophilic quartz or mica substrate in the 2 mg/mL SiTPy DMF solution. After one drop of 1 mol/L HCl was added,the DMF solution was heated at 80℃ overnight. The substrate was well washed with DMF and methanol to remove physically adsorbed SiTPy. The metalated complexes of SiTPy SAMs (SiTPyM,M = Fe2+ ,Zn2+ ,Eu3+ and Tb3+ ) were prepared by immersing the freshly prepared SiTPy SAMs (quartz or mica substrate) into the 10 mmol/L inorganic salt solutions for about 30 min,which was then well washed with water and methanol to remove unreacted inorganic salts. 2.4. Spectroscopic measurements and characterization
UV-vis spectra were measured with the use of Shimadzu UV-2550 UV-vis spectrophotometer. Thermogravimetric (TG) analysis was performed on Perkin-Elmer TGA7,under a constant-flow air atmosphere from room temperature to 800℃ at a rate of 10℃/min. Fourier transform infrared spectra (FITR) were measured by using a Nicolet NEXUS 470 spectrometer,operating at a resolution of 0.5 cm-1 at 25℃.
Fluorescence spectra were recorded by using Shimadzu RF-5300PC spectrophotometer with the excited wavelengths for the pure SiTPy ligand at 259 nm,Zn2+ /Fe2+ -SiTPy complexes at 263 nm, Eu3+ -SiTPy at 337 nm and Tb3+ -SiTPy at 344 nm,respectively.
X-ray photoelectron spectra (XPS) of the SAMs were recorded by using a VGESCALAB MKII multifunction spectrometer,with non-monochromatized Mg-KaX-rays as the excitation source. The system was carefully calibrated by the Fermi-edge of Ni,Au 4F2/7, and Cu 2p2/3binding energy. A pass energy of 70 eV and a step size of 1 eV were chosen when taking spectra. In the analysis chamber, pressures of 1×10-7 Pa to 2×10-7 Pa were routinely maintained. The binding energies obtained in the XPS analysis were corrected by referencing the Cl peak to 284.60 eV.
Atomic force microscope (AFM) images were recorded using a Nanofirst 3600A atomic force microscope (Suzhou HZS-Nanosurf Nanotechnology Co.,Ltd.,China) by means of a tapping mode with a silicon cantilever having a force constant of 40 N/m and a resonance frequency of 300 kHz in air. The SAMs of SiTPy were assembled on the mica substrate surface. 3. Results and discussion 3.1. Preparation of silylated-terpyridine derivative
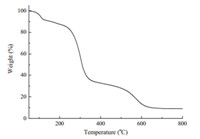
Multidentate ligand of Pyterpy was synthesized and purified based on the literature method [9, 10]. The white powders of Pyterpy was then dissolved in the nitromethane and reacted with excess 4-(chloromethyl)phenyltrimethoxysilane to form yellow gray silylated-terpyridine derivative of SiTPy (Fig. 1A). After dried in vacuo,the TG curve of SiTPy was measured from room temperature to 800℃. As shown in Fig. 2,the mass decrease process could be divided into three steps. The first step was about 10% mass decrease below 110℃,which was attributed to evaporation of the adsorbed water. The second step of mass decrease (nearly 70%) was the temperatures from 110℃to320℃,which may be attributed to the decomposition of the terpyridine and phenyl substituents of SiTPy. Thirdly,the mass decrease at the temperatures between 500℃and 600℃ could be attributed to the decomposition of the organic species covalently attached to the silicon. Finally,the residue (above 600℃) was about 10%,which may be attributed to the silicon dioxide produced at high temperature and in agreement with the chemical composition of the SiTPy.
|
Download:
|
| Fig. 2. hermogravimetric curve of SiTPy under air atmosphere. | |
FTIR spectrum of SiTPy showed several typical bands at the wavenumbers of about 3430,3044,2924,2843,1673,1442-1388 and 1124 cm-1 . The broad band at about 3430 cm-1 was designated to thev(OH) stretching vibration of hydroxyl groups of the adsorbed water [11]. Peaks at 3044,2924 and 2843 cm-1 were attributed to thevasandvs(CH) vibrations of -CH3and -CH2substituents on the trimethoxysilane. The peak centered at 1673 cm-1 was assigned to the pyridine and terpyridine substituents. In addition,the bands at 1530 and 1442-1388 cm-1 were associated with the C55NandC55C stretches of the terpyridine moiety. Finally,the peak at about 1124 cm-1 was designated tor(Si-O-CH3) and v(Si-O) vibration of the silane substituents [7]. 3.2. Absorbance of SiTPy and its metalated complexes
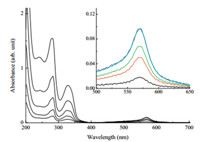
The absorption behaviors of SiTPy ligand and its metalated complexes with four transition metal ions of Fe2+ ,Zn2+ ,Eu3+ and Tb3+ were studied in the methanol solutions. The Fe2+ -SiTPy complex was purple,the other metalated complexes investigated were colorless. As an example,Fig. 3 shows absorption spectra for the mixtures of the ligand SiTPy with Fe2+ ions in the molar ratios from 2:1 to 1:8 in the methanol solutions. The ligand-basedp-p* and π-p* transitions for the pure SiTPy ligand appeared at about 241,282 and 326 nm [4]. These peaks shifted,split or weakened when the ligand was coordinated with the transition metal ions. Different from the pure ligand and the other metalated complexes, the Fe2+ -SiTPy complex has an absorption band at about 570 nm, which was attributed to the metal-ligand charge transfer (MLCT) process. When the molar ratios of the Fe2+ ions relative to the ligand increased to about 1:4,the absorption intensity of this MLCT reached to the maximum value due to all Fe2+ ions have been coordinated with the SiTPy ligand.
|
Download:
|
| Fig. 3. Absorption spectra for the mixtures of the ligand SiTPy with Fe2+ ions in the molar ratios from 2:1 to 1:8 in the methanol solutions. Inserted graph: enlarged absorption spectra between 500 nm and 650 nm. (Bottom up: 2:1,1:1,1:2,1:4 and 1:8. Concentration of Fe2+ ion: 1.0×10-4 mol/L.). | |
The fluorescence of the ligand SiTPy was concentration dependent. Fig. 4A shows the emission spectra for the SiTPy in the methanol solutions with its concentrations ranged from 1.0×10-6 mol/L to 5.0×10-4 mol/L (excited at 259 nm). The excited wavelength was determined based on the excitation spectrum of the ligand. These curves revealed a broad emission band centered at about 456 nm. Almost no change was recorded for the emission wavelength,but the emission intensity was strongly dependent on the ligand concentrations; that is,it became stronger when the ligand concentrations increased from 1.0×10-6 mol/L to 1.0×10-5 mol/L,while it became weaker and weaker when the concentrations further increased from to 1.0×10-5 mol/L to5.0×10-4 mol/L. Very weak emission was recorded when the SiTPy concentration reached to 1.0×10-3 mol/L. Based on the emission spectra,we can find that the strong concentration quenching occurred when the SiTPy concentrations were over 1.0×10-5 mol/L.

|
Download:
|
| Fig. 4. (A) Emission spectra for the SiTPy in the methanol solution with its concentrations ranged from 1.0×10-6 mol/L to 5.0×10-4 mol/L (excited at 259 nm). (B) Emission spectra for the Zn2+ -SiTPy complexes in the methanol solution with the molar ratios of Zn2+ /SiTPy from 1:0.5 to 1:8,excited at 263 nm. (Concentration of Zn2+ ion: 1.0×10-6 mol/L.) | |
The excited energy of organic ligands could be transferred to some metal ions coordinated or not. Here,four transition metal ions (Zn2+ ,Fe2+ ,Eu3+ and Tb3+ ) were selected and added to the SiTPy methanol solution to form its metalated complexes,the emission behaviors both from the SiTPy ligand,Zn2+ /Fe2+ -SiTPy complexes and from the central ions of Eu3+ and Tb3+ were measured and characterized. Fig. 4B shows the emission spectra for the Zn2+ -SiTPy complex in the molar ratios of Zn2+ /SiTPy ranged from 1:0.5 to 1:8,excited at 263 nm. The Zn2+ concentration was kept at about 1.0×10-6 mol/L. The excitation spectrum was similar to that of the pure SiTPy. Broad emission bands were observed at about 452 nm,which was very similar to that of the pure SiTPy solution. The results indicated that the transition metal ions like Zn2+ have almost no influence on the fluorescence of the ligand [12].
Different from the emission features of the pure ligand and its Zn(II)-metalated complex,energy absorbed by the SiTPy ligand could be transferred to the Eu3+ and Tb3+ ions,resulting in the unique fluorescence emissions of the lanthanide ions. When these lanthanide ions were added into the SiTPy solution,the emissions from both ligand and central metal ions were observed; the relative intensity of which slightly related to the excited wavelengths.
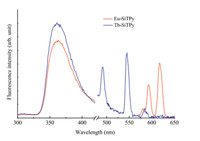
Fig. 5 shows the emission spectra for the Eu3+ /Tb3+ -SiTPy complexes (molar ratios,1:2),excited at 259 nm and 344 nm, respectively. It can be found that when the complexes were excited at 259 nm,emission spectra were mainly composed of the ligand emission at 363 nm; that is,under such a condition,the excited energy of the ligand of SiTPy was not so efficiently transferred to the excited state of the center lanthanide ions. While,when the complexes were excited at 344 nm,typical emission peaks corresponding to Eu3+ and Tb3+ were recorded.
|
Download:
|
| Fig. 5. Emission spectra for the Eu3+ /Tb3+ -SiTPy complexes (molar ratios,1:2) in the methanol/dichloromethane. For the ligand emission (300-450 nm) excited at 259 nm,for the Eu3+ and Tb3+ emission (450-650 nm) excited at 337 nm and 344 nm,respectively. | |
In details,for the Tb3+ -SiTPy complex,the band at around 363 nm was the ligand emission,and peaks between 450 and 650 nm were characteristic of Tb3+ emissions. These peaks were designated to the transitions of5 D4→7F3(621 nm),5 D4→7F4 (582 nm),5 D4→7F5(544 nm),5 D4→7F6(489 nm),respectively [13]. For the Eu3+ -SiTPy complex,besides the ligand emission at about 363 nm,another two emission peaks appeared at around595 and 615 nm,which were designated to be5 D0→7F1and5 D0 7F2!transitions,respectively [13]. Compared to the Eu3+ -SiTPy complex,much stronger Tb3+ emissions were recorded for the Tb3+ -SiTPy complex,indicating that the excited energy of SiTPy ligand could be more efficiently transferred to the central Tb3+ ions.
A comparison of the fluorescence emission for the SiTPy ligand, its Zn2+ /Fe2+ metalated complexes with that of the lanthanide complexes could find that,the ligand emission in the lanthanide complexes appeared at about 363 nm,largely ‘‘blue’’ shifted as compared with that in the Zn2+ /Fe2+ complexes. Such a difference may be attributed to the reason that the energy of the exited state of Tb3+ (5 D4) ion was 20.6×103 cm-1 and that of Eu3+ ion (5 D0) was 17.3×103 cm-1 ,respectively [13, 14],which is very closely to that of the excited state of the ligand emission at 456 nm (21.9×103 cm-1 ). Hence,the energy at this excited state of the SiTPy ligand is largely transferred to the excited states of the Tb3+ or Eu3+ ions. 3.4. Self-assembled monolayers of SiTPy and its metalated complexes at interfaces
The present SiTPy ligand has a silane substituent of (CH3O)3Si- that can react with hydroxyl group to form silylated-terpyridine functionalized materials [7]. Here,the freshly prepared hydrophilic quartz or mica substrate was immersed in the DMF solution of SiTPy or its metalated complexes for the formation of SAMs of SiTPy and M-SiTPy (M was Fe2+ ,Eu3+ and Tb3+ ). Absorption spectrum of Fe-SiTPy SAM showed an absorption band at about 600 nm (curve not shown) due to the metal-ligand charge transfer process. This band slightly red shifted as compared with that in the methanol solution (Fig. 3),the phenomenon of which has been well occurred for the dyes in their molecular aggregates and thin films due to a strong molecular coupling interaction [15].
Element compositions for the (M-)SiTPy SAMs were detected by using the XPS technique. First,three peaks were obtained for the SAMs of SiTPy except for the Si element from substrate surface. The binding energy of these three peaks was as follows: 284.6,399.4- 403 and 532.6 eV,which could be assigned to the elements of C(1s),N(1s) and O(1s),respectively. The results were in agreement with the composition of SiTPy SAMs. For the metalated MSiTPy SAMs,besides three elements of C,N and O,another element of metal ion was detected. For instance,the binding energy for the elements of Fe(II) and Eu(III) was about 707-720 and 1133- 1163 eV,respectively. Thus,these XPS data confirmed formation of the SiTPy SAMs and their metalated complexes at interfaces.
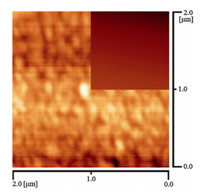
Morphology of the SiTPy SAMs was characterized by using AFM technique. Fig. 6 shows an AFM image of the SiTPy SAM on the mica substrate surface together with that of naked mica. These photos revealed a smooth image for the clean mica (the inserted photo), while many irregular domains were observed for the SiTPy SAM. The sizes for the domains were in the range of tens to hundreds of nanometers,with the average height of the monolayers below 2 nm. No significant difference was observed for the AFM images before and after the formation of the metalated SiTPy SAMs,which was reasonable because coordination of the small transition metal ions with the TPy substituent could not cause obviously structural difference.
|
Download:
|
| Fig. 6. AFM image for the SAMs of SiTPy on the mica substrate surface. (Inserted image: naked mica substrate.) | |
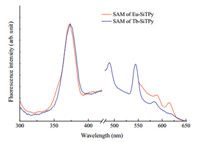
Finally,the fluorescent emission spectra for the Eu3+ and Tb3+ -SiTPy SAMs were measured and shown in Fig. 7. Similar to those observed in the solutions,two groups of the emission bands were recorded,one appeared at about 370 nm corresponding to the emission from the ligands and another one appeared at the range from 450 to 650 nm corresponding to the emissions from either Tb3+ (5 D4→7F3-6)orEu3+ (5 D0→7F1,2) ions. The relatively higher emission intensity was also observed for the Tb3+ -SiTPy SAM as compared with that for the Eu3+ -SiTPy SAM,which could also be attributed to the reason that excited energy of SiTPy ligand could be more efficiently transferred to the central Tb3+ ions.
|
Download:
|
| Fig. 7. Emission spectra for the SAMs of the Eu3+ /Tb3+ -SiTPy complexes. For the ligand emission (300-450 nm) excited at 259 nm,for the Eu3+ and Tb3+ emission (450-650 nm) excited at 337 and 344 nm,respectively. | |
We have demonstrated preparation of a silylated-terpyridine derivative,its metalated complexes and self-assembled monolayers. This ligand itself showed strong emission at about 456 nm. The excited energy of the ligand can be transferred to the lanthanide ions of Tb3+ and Eu3+ leading to strong emissions characteristic of lanthanide ions. This ligand and its metalated complexes can further form SAMs on the hydrophilic substrate surfaces. Considering about the unique structural features of the compound,we suggest that this ligand and its metalated complexes can be used as building blocks to construct supramolecular functional materials and molecular devices.
AcknowledgmentThis work was supported by National Natural Science Foundation of China (No. 21373058) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1117).
| [1] | A. Wild, A. Winter, F. Schlütter, U.S. Schubert, Advances in the field of π-conjugated 2,2':6',2""-terpyridines, Chem. Soc. Rev. 40 (2011) 1459-1511. |
| [2] | S.C. Yuan, H.B. Chen, H.C. Wang, Assembly and metal complex properties of terpyridine ligands, Prog. Chem. 21 (2009) 2132-2152. |
| [3] | N. Tuccitto, I. Delfanti, V. Torrisi, et al., Supramolecular self-assembled multilayers of terpyridine-functionalized perylene bisimide metal complexes, Phys. Chem. Chem. Phys. 11 (2009) 4033-4038. |
| [4] | C. Mugeman, J.F. Gohy, C.A. Fustin, Functionalized nanoporous thin films from metallo-supramolecular diblock copolymers, Langmuir 28 (2012) 3018-3023. |
| [5] | C. Haensch, M. Chiper, C. Ulbricht, et al., Reversible supramolecular functionalization of surfaces: terpyridine ligands as versatile building blocks for noncovalent architectures, Langmuir 24 (2008) 12981-12985. |
| [6] | C.F. Zhang, H.X. Huang, B. Liu, M. Chen, D.J. Qian, Spectroscopic study on the 4'-(4-pyridyl)-2,2':6',2""-terpyridine and its metal complexes, J. Lumin. 128 (2008) 469-475. |
| [7] | A.P. Duarte, M. Gressier, M.J. Menu, et al., Structural and luminescence properties of silica-based hybrids containing new silylated-diketonato europium(III) complex, J. Phys. Chem. C 116 (2012) 505-515. |
| [8] | Q.F. Jin, G.X. Liao, S.J. Yu, X.G. Jian, Characterization and tribological investigation of self-assembled trimethoxysilyl-functionalized poly (phthalazinone ether ketone) thin films on glass substrates, Chin. Chem. Lett. 21 (2010) 973-975. |
| [9] | J.H. Wang, G.S. Hanan, A facile route sterically hindered and non-hindered 4'-aryl-2,2':6',2""-terpyridines, Synlett 8 (2005) 1251-1254. |
| [10] | L. Hou, D. Li, W.J. Shi, Y.G. Yin, S.W. Ng, Ligand-controlled mixed-valence copper rectangular grid-type coordination polymers based on pyridylterpyridine, Inorg. Chem. 44 (2005) 7825-7832. |
| [11] | Z. Naseri, A.N. Kharat, A. Banavand, A. Bakhoda, S. Foroutannejad, First now transition metal complexes of thienyl substituted terpyridine: structural, photophysical and biological studies, Polyhedron 33 (2012) 396-403. |
| [12] | P.F. Shi, Q. Jiang, H.C. Duan, D.Q. Wang, Synthesis, characterization and cytotoxicity of fluorescent organotin complexes of terpyridine derivatives, Chin. Chem. Lett. 25 (2014) 586-588. |
| [13] | X.M. Xiang, D.J. Qian, H.G. Liu, H.T. Chen, X.S. Feng, Fabrication of europium complexes with 4'-(4-methylphenyl)-2,2':6',2""-terpyridine and 4,40-dinonyl-2,2'-dipyridyl at the air-water interface and their emission properties in Langmuir-Blodgett films, Colloids Surf. A: Physicochem. Eng. Asp. 273 (2006) 29-34. |
| [14] | T. Ala-Kleme, M. Latva, K. Haapakka, Study on the radiative 5D4→7Fj relaxation dynamics of Tb(III) in electrochemically excited self-assembled dimeric heterodinuclear Tb(III)-Ln(III) chelates, Anal. Chim. Acta 403 (2000) 161-171. |
| [15] | H.G. Liu, X.S. Feng, L.J. Zhang, et al., Influences of hydrophilic and hydrophobic substituents on the organization of supramolecular assemblies of porphyrin derivatives formed at the air/water interface, Mater. Sci. Eng. C 23 (2003) 585-592. |




