b State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China
The textile industry has been traditionally treated as a pillar industry in China,which contributes greatly to the national economy [1]. Over 10% of the gross domestic product came from the textile industry in the last decade [2]. Nevertheless,the fact that the textile industry consumes a large amount of water resources is undeniable and,usually,almost every process of the textile industry (pretreating,sizing,dyeing,scouring,bleaching and finishing) can generatewastewater.Manypollutants,includingunconsumeddyes, polymers,fibers,greases,heavy metals and salts,are washed from the textile material and discharged with wastewater [3]. It is reported that during the dyeing process,about 10%-15% of the dyes do not adhere to the fibers and thus are discharged into the aquatic environment [4]. Besides esthetic problems [5],these dyes,together with the toxic substances,would also bring other serious threats to the environments and human health.
Classical methods for the treatment of textile wastewater mainly refer to the combinations of physical [6, 7],chemical and biological processes,which cause numerous problems during fullscale operation. One of the disadvantages of the classical methods is the generation of sludge and other solid waste [8, 9]. In other cases,secondary pollution would occur when some chemical oxidants are added into the water. Additionally,traditional means of dealing with the textile wastewater do not yield satisfying results,particularly in the decolorization of the wastewater,since some dyes have recalcitrant characteristics and are non-biodegradable by the conventional activated sludge process [10, 11, 12].
Advanced oxidation processes (AOPs) are promising and effective to remove the color from the textile wastewater. Various industrial wastewater that contain toxic and recalcitrant pollutants can be treated via AOPs due to its highly powerful oxidizing ability [13, 14, 15, 16]. Moreover,the AOPs have greatly shortened the reaction time of the treatment process without the generation of additional sludge,thus saving operational costs. Ozonation has been proven to be one of the effective and environment-friendly ways to accomplish the task of color removal in textile wastewater among all the AOPs [17]. Many kinds of dyes can be decolorized by ozone except nonsoluble,disperse dyes [18]. Ozonation is regarded to have a combined effect of molecular ozone and the hydroxyl radical (·OH),depending on the pH of the solution [19]. The efficiency of ozonation depends on several factors,such as the initial dye concentration,ozone dosage and pH value of the solution [17, 19, 20]. Ozone can also be used in combination with other biological processes because some biorefractory dyes,owing to the oxidation of ozone,would be converted into biodegradable substances [21]. The BOD5/COD ratio is used to assess whether biological methods can be introduced. Research shows that the BOD5/COD ratio has been improved significantly by the ozonation process and the textile wastewater pollutants become biodegradable, therefore the use of biological treatment is a viable addition [22, 23]. However,a study on the ozonation products and the degradation pathway is still rare.
According to the chemical structures,dyes can be classified as groups of azo,anthraquinone,triarylmethane,phtalocyanine,etc. [24]. Among all kinds of dyes,the azo dye is one that has many analogs and has been widely used. Over 50% of the commercial dyes used in textile industry are azo dyes [19]. There are two key components in the dye molecules,the chromophores and the auxochromes. The chromophores refer to the unsaturated groups of -N=N-,which are difficult to treat biologically [25]. On the other hand,the auxochromes enhance the intensity of the color and make the dye soluble in water and easy to attach to fibers [19, 26, 27]. Since a large number of dyes are washed out into the wastewater in textile industry and many azo dyes have been proved to be hazardous to human health,it is quite important and necessary to use an effective method,such as AOPs,to remove the dye residues from wastewater.
Despite the strong oxidation ability of AOPs,the oxidation products may have high toxicity that have not yet been well studied and might be easily ignored [18, 28]. Besides,some inorganic salts [29, 31] have not been the focus of much research on the ozonation products of dyes [25, 28]. Therefore,it is of great importance to characterize the ozonation products and explore the possible degradation pathway for better understanding and control of the ozonation process.
The aim of this study was to apply ozonation to the decolorization and degradation of the azo dye,C.I. Reactive Red 195 (RR195). The kinetics of ozonation were investigated and several factors influencing the efficiency of ozonation were analyzed to determine the optimization parameter based on the reaction rate constant. At the same time,the major ozonation products generated during ozonation were identified. The possible degradation pathway of the RR195 by ozonation is proposed.
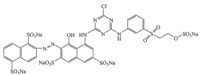
2. Experimental 2.1. ChemicalsC.I. Reactive Red 195 (RR195,CAS Registry No. 93050-79-4, C31H19O19N7S6Na5Cl,λmax = 543 nm) was supplied by a textile factory in Quanzhou,Fujian Province,China and used without further purification. Fig. 1 shows the chemical structure of RR195. Sodium carbonate and sodium chloride were analytical grade (Xilong Chemical Co.,Ltd.,Guangdong,China). Dye solutions were prepared with tap water in order to simulate the true textile wastewater.
|
Download:
|
| Fig. 1. Chemical structure of C.I. Reactive Red 195. | |
The schematic of the equipment is shown in Fig. 2. The ozonation was carried out in a semi-batch,bubble reactor with a volume of 15 L. The ozone gas was produced by a commercial ozone generator (YG-30,Xuzhou City Tianlan Ozone Equipment Co.,Ltd.,Jiangsu,China) with a gaseous flow meter (LZB-6, Tianchuan Instrument Factory,Shanghai,China). Pure oxygen gas from a pressure cylinder was fed into the ozone generator. A peristaltic pump (BT00-300T,Baoding Longer Precision Pump Co., Ltd.,Hebei,China) was used to deliver samples from the reactor. Plastic tubing was used to connect the ozone generator and the aerators in the reactor and silicone tubing was used connecting the reactor and the pump. Surplus ozone was passed into a gas trapping bottle containing potassium iodide solution.

|
Download:
|
| Fig. 2. Schematic of the semi-batch reactor. | |
The concentration of RR195 in aqueous solution was determined via a UV-vis spectrophotometer (723Pc,Shanghai Spectral Equipment Co. Ltd.,Shanghai,China) with a 10 mm cuvette at the maximum absorption wavelength of 543 nm. The pH was adjusted with hydrochloric acid or sodium hydroxide and measured with a pH meter (pH-25,Rex Analytical Instrument Co.,Ltd.,Shanghai, China). The dissolved ozone concentration was analyzed by the iodometric method [31].
The ozonation products were analyzed using an ultraperformance liquid chromatography-tandem mass spectrometer (LC-MS/MS) (LC,Agilent 1290 Infinity; MS,Agilent 6490 QQQ mass spectrometer,Agilent Co.,Ltd.,USA) in the ESI+ mode. The LC was equipped with a C18 column (Kromasil,250 mm × 4.6 mm, particle size 5 μm,AkzoNobel Co.,Ltd.,Sweden). The gradient elution was performed with a mixture of methanol and water,and the mobile phase flow rate was 1.0 mL/min. Both solvents were previously degassed with an ultrasonic device. The gradient program started at 99% of water and 1% of methanol for 2 min. The proportion of methanol was then increased to 10% over the next 15 min,and subsequently increased to 25% over 8 min. All the samples were filtered with 0.22 mm membrane before being injected into LC-MS/MS at a volume of 20 μL.
2.4. ProceduresThe ozonation efficiency was calculated according to the equation below.
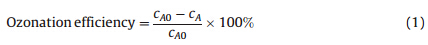
Because of the low solubility of ozone gas in water [32],it is necessary to estimate the variation of ozone concentration in the liquid phase with time and the mass transfer conditions of ozone. The mass transfer coefficient (kLa) represents the mass transfer rate of a gas transferring from gas phase to liquid phase,according to the double-layer model. For the mass transfer coefficient of ozone,it can be determined using the following equation.


Several parameters,including initial dye concentration,flow rate of ozone,initial pH of the solution and dyeing auxiliaries, which influenced the rate of decolorization,were studied to optimize the working conditions. The impacts were evaluated with the rate constant (k). Assuming that the decolorization process followed the pseudo-first-order model,the rate constant could be described using the equation below.

Integration of both sides of Eq. (4) eventually furnishes the following equation.

All the experiments were performed at room temperature and only one parameter was altered in any one experiment. The dye was dissolved in tap water and the solution was added to the reactor before ozonation.
The ozonation process in the reaction included two steps. First, ozone gas was forced to be dissolved in the dye solution (step of mass transfer); and second,the ozone molecules and the chain reaction hydroxyl radicals in water reacted with dyes. The mass transfer coefficient (kLa) and the pseudo-first-order rate constant (k) were compared to decide the rate-determining step of the ozonation process.
RR195 was chosen as a model dye and a 100 mg/L dye solution was prepared for the study of ozonation products. A 5 mL volume of sample was taken from the reactor after 30 min ozonation. Mass spectra were extracted from the TIC chromatogram in the full-scan mode at certain retention times. Interpretations were carried out based on the mass spectra corresponding to the different mass-tocharge ratios,and the possible degradation pathway of the ozonation of RR195 was postulated.
3. Results and discussion 3.1. Ozone mass transfer in the semi-batch reactorThe variation of ozone concentration in the reactor with respect to time is shown in Fig. S1 in Supporting information. The operational flow rate of ozone gas was 3.47 L/min. The ozone concentration rose rapidly in the first 10 min from 0 to 11.2 mg/L and then reached saturation. Based on Eqs. (2) and (3),the mass transfer coefficient was calculated as 0.43 min-1.
3.2. Influence of operational parameters on ozonation 3.2.1. Effect of pHA series of dye solutions at different pH values of 2.8,7.9 and 10.1 was prepared to study the effect of initial pH on the decolorization in the unbuffered system. The pH of the untreated RR195 solution was about 8. The result is shown in Fig. S2 in Supporting information. It is obvious from the results that a 99% decolorization efficiency was reached in both acid and alkaline conditions in 15 min,which implied that the ozonation was quick and effective to remove the color of the dye. These results are in agreement with literature reports [9, 19, 20]. From the results,it could also be noticed that the degree of decolorization decreased as initial pH increased. This was due to the different dominant oxidant under different pH conditions. The hydroxyl radical was more likely to be produced in alkaline condition and inhibited in acid conditions [20]. The fact that the degree of decolorization decreased at higher pH indicated that RR195 was oxidized by ozone molecules rather than hydroxyl radicals.
It is also found that the pH of the dye solution decreased rapidly during ozonation with respect to time when the initial pH was in the basic range,but remained almost steady when the initial pH was 2.8 (Fig. S3 in Supporting information). Based on the previous reports [28, 30, 33],possible explanations for the decreasing of pH may be the consumption of hydroxyl ions and the production of organic and inorganic acids.
3.2.2. Effect of initial dye concentrationThe ozonation efficiency could also be affected by the initial dye concentration. An experimentwas performed at different initial dye concentrations of 100 mg/L,200 mg/L and 400 mg/L. The result is plotted in Fig. S4 in Supporting information. For the 100 mg/L and 200 mg/L dye solutions,less than 30 min was needed to remove almost all the color. However,it took nearly an hour to reach 99% of color removal in the 400 mg/L dye solution. It was noticeable that the ozonation efficiency decreased as the initial dye concentrations increased. The reason was due to the insufficient availability of ozone at the initial,elevated dye concentration and thus requiring more ozonation time to complete the decolourization.
3.2.3. Effect of ozone dosageColor removal of the RR195 solution as a function of the ozonation time was studied with different ozone flow rates (1.73 L/min, 3.47 L/min 5.20 L/min and 6.94 g/min). The results are shown in Fig. S5 in Supporting information. In most cases the decolorization was completed in about 10min with the ozone flow rates. The color removal efficiency increased as the ozone flow rate increased,which was consistentwith the theory of mass transfer. Based on the doublelayer theory,the driving force for the transfer of ozone gas to the liquid phase increases when the ozone gas concentration increases and,subsequently,leading to an enhancement of the ozone concentration in the dye solution and decolorization rate [34].
3.2.4. Effect of dyeing auxiliariesIn actual practices in the textile industry,many chemicals are added as dyeing auxiliaries to provide better dyeing effect [28, 34]. However,some of the auxiliaries act as radical scavengers in the wastewater,which can reduce the production of hydroxyl radicals. In this study,sodium carbonate and sodium chloride were added to the dye solution,separately,to determine the influence of a radical scavenger on ozonation,since these two kinds of salts are the most common auxiliaries used in textile industry and are both radical scavengers.
The effect of auxiliaries on the decolorization is illustrated in Fig. S6 in Supporting information. An increase in the degree of decolorization was observed when sodium chloride existed in the dye solution. This was because the radical scavenger reduced the transfer of ozone to hydroxyl radical,resulting in more ozone molecules taking part in the decolorization. The results further proved that RR195 was more easily removed by ozone. The pH of the dye solution reached about 10.7 after adding sodium carbonate,and then the pH was adjusted to 7.9 with hydrochloric acid. However,the decolorization rate with sodium carbonate at this pH was almost the same as that without sodium carbonate in the dye solution. The results of statistical analysis showed that the rate of decolorization with sodium chloride in the dye solution presented significant difference (P = 0.021,<0.05) from that without auxiliaries. The same result was determined (P = 0.028, <0.05) when sodium carbonate existed in the dye solution without pH adjustment. However,there was no significant difference (P = 0.883,>0.05) when sodium carbonate existed in the dye solution with pH adjustment compared to that without auxiliaries. The possible reason for the result was due to the conversion of the carbonate ion into bicarbonate ion at about pH 8,and bicarbonate ion was not a radical scavenger. Another experiment was performed without the adjustment of pH after adding sodium carbonate. It was clear from the result that the decolorization degree increased when the carbonate ion existed.
3.2.5. The optimization for ozonationThe pseudo-first-order rate constants under different operational conditions were calculated and the results are shown in Table S1 in Supporting information. It could be deduced that the difference of k values in acid and initial pH (7.9) conditions were not significant. Therefore,the untreated dye solution was used without adjustment of pH for the following research. The pseudofirst- order rate constants presented a decreasing tendency as the ozone flow rate dropped. In the view of energy savings,a flow rate of 3.47 L/min was chosen. Since the mass transfer coefficient of ozone was 0.43 min-1,it could be indicated from the table in the Supporting information that the values of pseudo-first-order rate constants had the same order of magnitude with the mass transfer coefficient,which meant the rate-determining step could be either the mass transfer process or the decolorization process. In other words,the entire ozonation process was determined by both mass transfer process and reaction.
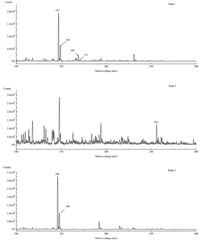
3.3. Identification of ozonation products in ozonationReferring to the results of previous research [25],analysis of the ozonation products of RR195 was carried out. The sample was taken from the solution of initial dye concentration of 100 mg/L and ozonation time of 30 min. Dyeing auxiliaries had great influence on decolorization,thus were excluded for the study of ozonation products and degradation pathway. The total ion current (TIC) chromatogram of LC-MS/MS for the sample is illustrated in Fig. 3. The peaks 1-3 in Fig. 3 could be possibly regarded as the ozonation products of RR195. The mass spectra of peaks 1-3 are shown in Fig. 4. The typical mass-to-charge ratios (m/z) of each mass spectrum and their interpretation are listed in Table 1. The compound in peak 1 containing fragment A1 with a short retention time had stronger polarity,while the compound in peak 3 containing A4 had the longest retention time due to the symmetrical and nonpolar structure. From the interpretation,it could be inferred that A1 and A4 had the similar structure as triazines. The structure of A3 was quite similar to aromatic sulfonates. However,not all the mass-to-charge ratios could be interpreted due to the possible impurities from the tap water (as the solvent) and commercial dye RR195,as well as the limited knowledge.

|
Download:
|
| Fig. 3. TIC chromatogram in the full-scan mode for ozonation products. | |
|
Download:
|
| Fig. 4. Mass spectra of peaks 1-3 (retention time: peak 1,11.2 min; peak 2,12.3 min; peak 3,16.4 min). | |
| Table 1 Peaks with mass-to-charge ratios and their interpretation. |
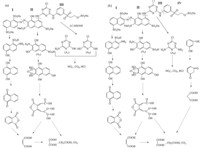
Two possible degradation pathways for ozonation of RR195 were preliminarily proposed based on the compositions of peaks 1-3,and are presented in Fig. 5(a) and (b). The substances existing in solution were the mix of products from two degradation pathways. Degradation pathway No. 1 in Fig. 5(a) contained compounds with fragments A1,A2 and A3,and pathway No. 2 contained compounds with fragments A2 and A3. Since the decolorization efficiency reached nearly 100%,the -N=N- bond must have been broken during the ozonation process. By the synergistic effect of ozone and the hydroxyl radical,the RR195 was broken into several fragments. Parts of the ozonation products would finally be fully decomposed by carbon dioxide and other small organic acids if the oxidation continued.
|
Download:
|
| Fig. 5. Proposed degradation pathway of RR195 with ozone. (a) Degradation pathway No. 1; (b) degradation pathway No. 2 (the solid lines refer to the proposed degradation pathway and the dotted lines indicate that part of the ozonation products might be further degraded to other smaller compounds). | |
The ozonation treatment of RR195 was proved to be effective in color removal with an efficiency of almost 100%. Results showed that several important factors,such as initial pH and dye concentration in solution,ozone gas flow rate and dyeing auxiliaries,could influence the efficiency of decolorization. The color removal of RR195 was enhanced as pH decreased,or the dyeing auxiliaries,sodium carbonate and sodium chloride,were added to the dye solution,which attributed to the reinforcing of ozone molecule. The results also showed that the degree of decolorization was primarily proportional to the ozone flow rate. More reaction time was needed to reach the complete decolorization when the initial dye concentration increased.
The ozonation products were partially characterized by the LC-MS/MS data and the possible degradation pathway was proposed. According to the proposed degradation,the dye molecule of RR195 could be broken into small organic acids, sulfate and nitrate. However,some organic compounds containing the structure of benzene,naphthalene and triazine species existed. This indicated that although the degradation of RR195 was completely achieved,some aromatic and triazine compounds would still remain in the wastewater,whichmay be hazardous to the environment and human health [35]. Even so,dyewastewater treated by ozonation could yield more biodegradable byproducts, thus the ozonation could be applied as a pretreatment technique.
AcknowledgmentsThe authors would like to thank Qitong Yi of the College of the Environment&Ecology,XiamenUniversity forhelpinguse of LC-MS/ MS. Prof. John Hodgkiss is thanked for assistance with the English.
Appendix A. Supplementary dataSupplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.10.024.
| [1] | H.L. Lin, H.Y. Li, C.H. Yang, Agglomeration and productivity: firm-level evidence from China's textile in-dustry, China Econ. Rev. 22 (2011) 313-329. |
| [2] | J. Chen, Q. Wang, Z. Hua, G. Du, Research and application of biotechnology in textile industries in China, Enzyme Microb. Technol. 40 (2007) 1651-1655. |
| [3] | C. Hachem, F. Bocquillon, O. Zahraa, M. Bouchy, Decolourization of textile industry wastewater by the photocatalytic degradation process, Dyes Pigments 49 (2001) 117-125. |
| [4] | H. Ali, S.K. Muhammad, Biodecolorization of acid violet 19 by Alternaria solani, Afr. J. Biotechnol. 7 (2008) 831-833. |
| [5] | L. Núñez, J.A. García-Hortal, F. Torrades, Study of kinetic parameters related to the decolourization and mineralization of reactive dyes from textile dyeing using Fenton and photo-Fenton processes, Dyes Pigments 75 (2007) 647-652. |
| [6] | G. Masoud, R. Roohan, M.A. Mohammad, Removal of methylene blue by tea wastages from the synthesis waste waters, Chin. Chem. Lett. 22 (2011) 225-228. |
| [7] | H. Yao, X.M. You, Q. Lin, et al., Multi-stimuli responsive metal-organic gel of benzimidazol-based ligands with lead nitrate and their use in removal of dyes from waste-water, Chin. Chem. Lett. 24 (2013) 703-706. |
| [8] | L.B. Chu, X.H. Xing, A.F. Yu, et al., Enhanced ozonation of simulated dyestuff wastewater by microbubbles, Chemosphere 68 (2007) 1854-1860. |
| [9] | M.H. Santana, L.M. Da Silva, A.C. Freitas, et al., Application of electrochemically generated ozone to the discoloration and degradation of solutions containing the dye Reactive Orange 122, J. Hazard. Mater. 164 (2009) 10-17. |
| [10] | S. Meriç, D. Kaptan, T.Ölmez, Color and COD removal from wastewater containing Reactive Black 5 using Fenton's oxidation process, Chemosphere 54 (2004) 435-441. |
| [11] | H.S. El-Desoky, M.M. Ghoneim, R. El-Sheikh, N.M. Zidan, Oxidation of Levafix CA reactive azo-dyes in industrial wastewater of textile dyeing by electro-generated Fenton's reagent, J. Hazard. Mater. 175 (2010) 858-865. |
| [12] | I. Arslan, I.A. Balcioglu, D.W. Bahnemann, Heterogeneous photocatalytic treatment of simulated dyehouse effluents using novel TiO2-photocatalysts, Appl. Catal. B: Environ. 26 (2000) 193-206. |
| [13] | K. Chiang, R. Amal, T. Tran, Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide, Adv. Environ. Res. 6 (2002) 471-485. |
| [14] | J. Anotai, M.C. Lu, P. Chewpreecha, Kinetics of aniline degradation by Fenton and electro-Fenton processes, Water Res. 40 (2006) 1841-1847. |
| [15] | I. Kim, N. Yamashita, H. Tanaka, Performance of UV and UV/H2O2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan, J. Hazard. Mater. 166 (2009) 1134-1140. |
| [16] | M. Deborde, S. Rabouan, P. Mazellier, J.P. Duguet, B. Legube, Oxidation of bisphenol A by ozone in aqueous solution, Water Res. 42 (2008) 4299-4308. |
| [17] | L.W. Lackey, R.O. Mines Jr., P.T. McCreanor, Ozonation of acid yellow 17 dye in a semi-batch bubble column, J. Hazard. Mater. 138 (2006) 357-362. |
| [18] | P.C. Vandevivere, R. Bianchi, W. Verstraete, Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies, J. Chem. Technol. Biotechnol. 72 (1998) 289-302. |
| [19] | A. López-López, J.S. Pic, H. Debellefontaine, Ozonation of azo dye in a semi-batch reactor: a determination of the molecular and radical contributions, Chemosphere 66 (2007) 2120-2126. |
| [20] | T.Y. Chen, C.M. Kao, A. Hong, C.E. Lin, S.H. Liang, Application of ozone on the decolorization of reactive dyes - Orange-13 and Blue-19, Desalination 249 (2009) 1238-1242. |
| [21] | A. Lopez, G. Ricco, G. Mascolo, et al., Biodegradability enhancement of refractory pollutants by ozonation: a laboratory investigation on an azo-dyes intermediate, Water Sci. Technol. 38 (1998) 239-245. |
| [22] | X.J. Wang, X.Y. Gu, D.X. Lin, F. Dong, X.F. Wan, Treatment of acid rose dye containing wastewater by ozonizing - biological aerated filter, Dyes Pigments 74 (2007) 736-740. |
| [23] | Y. He, X. Wang, J. Xu, et al., Application of integrated ozone biological aerated filters and membrane filtration in water reuse of textile effluents, Bioresour. Technol. 133 (2013) 150-157. |
| [24] | J.Z. Li, Dyeing Industry Wastewater Treatment, Chemical Industry Press, Beijing, 1997 (in Chinese). |
| [25] | S. Song, H.P. Ying, Z.Q. He, J.M. Chen, Mechanism of decolorization and degradation of CI Direct Red 23 by ozonation combined with sonolysis, Chemosphere 66 (2007) 1782-1788. |
| [26] | A.C. Gomes, J.C. Nunes, R.M. Simões, Determination of fast ozone oxidation rate for textile dyes by using a continuous quench-flow system, J. Hazard. Mater. 178 (2010) 57-65. |
| [27] | R. Molinari, F. Pirillo, M. Falco, V. Loddo, L. Palmisano, Photocatalytic degradation of dyes by using a membrane reactor, Chem. Eng. Process. 43 (2004) 1103-1114. |
| [28] | C. Wang, A. Yediler, D. Lienert, Z. Wang, A. Kettrup, Ozonation of an azo dye C.I. Remazol Black 5 and toxicological assessment of its oxidation products, Chemosphere 52 (2003) 1225-1232. |
| [29] | M. Muthukumar, N. Selvakumar, Studies on the effect of inorganic salts on decolouration of acid dye effluents by ozonation, Dyes Pigments 62 (2004) 221-228. |
| [30] | M. Koch, A. Yediler, D. Lienert, G. Insel, A. Kettrup, Ozonation of hydrolyzed azo dye reactive yellow 84 (CI), Chemosphere 46 (2002) 109-113. |
| [31] | Ministry of Housing and Urban-Rural Construction of the People's Republic of China, Ozone Generator for Water and Waste Water Treatment, Ministry of Housing and Urban-Rural Construction of the People's Republic of China, Beijing, 2010 (in Chinese). |
| [32] | R. Rosal, A. Rodriguez, M. Zerhouni, Enhancement of gas-liquid mass transfer during the unsteady-state catalytic decomposition of ozone in water, Appl. Catal. A: Gen. 305 (2006) 169-175. |
| [33] | A.R. Tehrani-Bagha, N.M. Mahmoodi, F.M. Menger, Degradation of a persistent organic dye from colored textile wastewater by ozonation, Desalination 260 (2010) 34-38. |
| [34] | M.T.F. Tabrizi, D. Glasser, D. Hildebrandt, Wastewater treatment of reactive dyestuffs by ozonation in a semi-batch reactor, Chem. Eng. J. 166 (2011) 662-668. |
| [35] | S.J. Stohs, S. Ohia, D. Bagchi, Naphthalene toxicity and antioxidant nutrients, Toxicology 180 (2002) 97-105. |