b Hubei Collaborative Innovation Center for Processing of Agricultural Products, Wuhan Polytechnic University, Wuhan 430023, China;
c China National Center for Food Safety Risk Assessment, Beijing 100050, China
The desire to look attractive is universal. Many young people are trying every possible way to lose weight and keep fit. In the 1930s,2,4-dinitrophenol (2,4-DNP) had been used as an oral weight-control drug,since studies showed it can obviously increase the basal metabolic rate,but soon its use for this purpose was banned by the US FDA because of serious adverse effects,including hyperthermia,cataracts and death [1, 2]. Last year,an 18 year-old Indian student died in the UK after apparently taking the banned slimming drug (2,4-DNP) that was popular among bodybuilders to get rid of extra fat (http://www.dailymail.co.uk/ femail/article-2315433/Sarah-Houston-cause-death-Boiled-aliveinternet- slimming-pills-DNP.html). To date,there have been 62 published deaths in the medical literature attributed to 2,4-DNP [3]. Thus,developing a rapid,simple and selective analytical method is very important for monitoring of trace 2,4-DNP in biological samples to avoid adverse events.
Currently,several strategies have been reported for the determination of 2,4-DNP,such as high performance liquid chromatography (HPLC) coupled with different detectors [4],gas chromatography (GC) [5] and electrochemical sensor [6]. Although chromatographic analysis has good sensitivity and accuracy,they are not accessible in non-professional laboratories for routine analysis due to the high cost of equipment and the requirement of a skillful operator. For the electrochemical sensor,interference of structural analogs is unavoidable because of the absent of effective separation. To the best of our knowledge,few studies to date have developed a rapid and low cost method for fluorescence detection of 2,4-DNP. However,due to the lack of selectivity,a more highly selective and effective sample pretreatment method is required for the determination of 2,4-DNP.
Solid-phase extraction (SPE) has been proposed as a wellestablished method for sample cleanup and concentration at trace level [7, 8]. Regarding spin column extraction,procedures such as sample loading,washing,and elution of target analytes can be accomplished by simple centrifugation and multiple samples can be processed simultaneously in the same centrifuge. Thus,sample pretreatment by using the spin column has many advantages: it is simple to perform,requires a low-volume of extraction solvent,and does not involve evaporation [9, 10]. However,the main problem associated with spin column extraction packed with common stationary phases is the low selectivity for the analytes. Molecularly imprinted polymers (MIPs) with selective recognition cavities,compared with other recognition elements,possess many promising characteristics,such as low cost and easy synthesis,high stability to harsh chemical and physical conditions,and excellent reusability [11, 12]. Thus,MIPs have been recognized as the promising and innovative adsorbents of spin column in the determination of target molecules.
In this study,molecularly imprinted spin column extraction coupled with fluorescence detection has been developed for the determination of 2,4-dinitrophenol in serum samples. Compared with the reported method,this method exhibited a lower detection limit and excellent extraction efficiency. 2. Experimental
Hydrophilic MIPs were synthesized according to the literature [13]. Briefly,MIPs were synthesized using 2,4-DNP as a template,acrylamide and glycidyl methacrylate as functional monomers,trimethylolpropane trimethylacrylate as a cross-linker and 2,2-azobisisobutyronitrile as an initiator. Subsequently,polymeric particles were dispersed in perchloric acid solution (10%,w/w) to obtain a hydrophilic layer by opening the epoxide ring. Finally,the particles were filtered,washed with water at pH 7.0 and dried under vacuum overnight. Hydrophilic non-imprinted polymers (NIPs) were prepared in the same way without the addition of the template molecule. Human serum samples were kindly provided by a team of volunteers,which were stored at-20 ℃ until analysis. Serum samples were spiked with different concentration of the analytes and then interfering proteins were removed by precipitation and centrifugation (10,000 rpm,5 min). The hydrophilic MIPs were packed into empty spin column (GL Sciences,Tokyo,Japan) and then this column was installed into a microtube (2 mL) for sample loading and washing. Prior to extraction,conditioning using acetonitrile and an aqueous solution was carried out and the column was centrifuged at 5000 rpm for 1 min,respectively. The samples (1 mL) were then applied to the conditioned spin column and centrifuged at 5000 rpm for 2 min. The loading procedure could be repeated by 5 times to obtain a high enrichment factor. Subsequently,the spin column was rinsed with 1.0 mL of acetonitrile to remove the sample matrix and structural analogs by centrifugation. Finally,the spin column was installed into a new microtube,and 2,4-DNP were eluted with 0.5 mL of acetonitrile/ acetic acid (9/1,v/v). After evaporating the solvent under a nitrogen stream to dryness at 40 ℃,0.2 mL of sampling buffer (pH 9.0),composed of 10 mmol/L borate and 80 mmol/L sodium dodecylsulfate,were added to redissolve the residue.
Because nitrophenol compounds could enhance the fluorescence intensities of fluorescein,sampling buffer and 0.21 mmol/L fluorescein solution (5 μL) was transferred into 96-well plate and determined by TECAN Infinite 200 (Tecan,San Jose,CA). Enhanced fluorescence intensity of 2,4-DNP was represented as F = F1 - F0. Here,F1 and F0 were the fluorescence intensities of the systems with and without 2,4-DNP,respectively. The standard curve method was employed in quantification of trace amounts of 2,4- DNP in spiked serum samples. Furthermore,serum samples were analyzed using the proposed method and traditional LC-MS/MS method [4] to verify the performance of the developed method for 2,4-DNP detection. 3. Results and discussion
The MIPs are tailor-made,stable polymers with molecular recognition abilities,so that they are excellent materials for providing selectivity in sample preparation. The synthesized hydrophilic MIPs exhibited highly selective recognition for the template molecule (imprinting factor was 3.34) and the maximum adsorption capacity was 138.9 mg/g. For the phenol compounds,the acidity decreased in the order of 2,4-DNP (pKa = 3.96),4- nitrophenol (4-NP,pKa = 7.16),2-nitrophenol (2-NP,pKa = 7.17) and phenol (pKa = 9.89). Therefore,ion-pair interaction,in addition to hydrogen bonding,could be formed to enhance the recognition ability of hydrophilic MIPs for 2,4-DNP. It was shown that 4-NP,2- NP and phenol could be removed in the washing procedure,which provided an effective way for the sole determination of 2,4-DNP in serum samples.
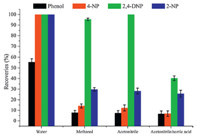
In this study,the extraction conditions were optimized by analyzing 0.1 μmol/L sample buffer. Firstly,the effect of solvent on the adsorption efficiency and recognition ability of hydrophilic MIPs-packed spin column was investigated (Fig. 1). The results indicated that nitrophenol compounds could be completely retained on the spin column using water as a solvent. Acetonitrile or methanol was unfavorable for the adsorption of 2-NP and 4-NP on the hydrophilic MIPs-packed spin column,because of the low polarity of nitrophenol compounds. Thus,water was used as the loading solution and acetonitrile was used as washing solution. Furthermore,addition of acetic acid solution could decrease the ion-pair interaction of the template-functional monomer,and then further decrease the adsorption efficiency of spin column for the 2,4-DNP. So,acetonitrile/acetic acid (9/1,v/v) was fixed as eluting solution in this study. Subsequently,volumes of washing and eluting solutions were also optimized (Fig. S1 in Supporting information). Under the optimized condition,the recovery of 2,4- DNP (0.01,0.1,1 μmol/L,respectively) on MIPs-packed spin column ranged from 89.5% to 92.1%. In addition,the spin column can be employed for 5 consecutive cycles without more treatment being required between cycles. The column is only needed to be preconditioned with acetonitrile and aqueous solution between extractions. The target molecules (2,4-DNP) were not detected in blank serum extracts from the reused spin column,indicating that there was no carryover effect.
|
Download:
|
| Fig. 1. The effect of solvent on the extraction efficiency of hydrophilic MIPs-packed spin column. | |
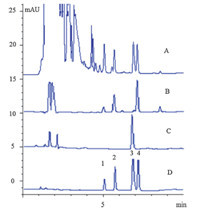
The results of chromatographic analysis indicated the sample matrix was removed and the nitrophenol compounds were retained on the spin column in the loading procedure (Fig. 2). In the washing step,it was interesting to note that phenol,4-NP and 2-NP were completely removed from the spin column using acetonitrile as the washing solution,which was beneficial for the sole detection of 2,4-DNP in serum samples and then the elimination of fluorescence interference. Due to the fluorescence quenching caused by the acetonitrile/acetic acid solution,the eluting solution was evaporated to dryness and redissolved using sampling buffer. The results demonstrated that an efficient cleaning and high recovery of 2,4-DNP were achieved by the hydrophilic MIPs extraction procedure,because of the highly specific recognition ability of MIPs.
|
Download:
|
| Fig. 2. The chromatograms obtained by direct injection of the spiked serum samples (1 μmol/L) (A),washing solution (B),eluting solution (C) and standard solution (D). Peaks: (1) phenol,(2) 4-NP,(3) 2,4-DNP,(4) 2-NP. HPLC-UV condition: SunFireTM C18 column (150 mm × 4.6 mmi.d.,particle size 5 μm,Waters,Milford,USA),acetonitrile/0.01 mol/L phosphate solution as the mobile phase and 279 nm as measurement wavelength. | |
The mechanism of indirect fluorescence detection can be described by the displacement of fluorescein by the nitrophenol compounds. Subsequently,the experimental condition of fluorescence detection was optimized (Fig. S2 in Supporting information). The pH of the sample buffer played an important role and the maximum signal intensities for 2,4-DNP were obtained at pH 9.0. Then the effect of the fluorescein concentration on the change of fluorescence intensities was studied. The optimized concentration was fixed at 0.21 μmol/L,which provided a relatively better signalto- noise ratio and high sensitivity.
Under the optimal conditions,analytical parameters such as the linear range,the correlation coefficient and the detection limit (LOD) were studied by analyzing spiked serum samples. It was shown that the change of fluorescence intensities against 2,4-DNP concentration was linear in the range of 2.5 nmol/L to 5000 nmol/L,and the correlation coefficient was 0.992. The relative standard deviation (RSD) for the measurement of each data point was less than 8.7%. The detection limit (LOD) was 1 nmol/L,which was lower than those of the reported method for 2,4-DNP detection using MIPs as the sorbents (40,50 and 400 nmol/L,respectively [6, 14, 15]). Assay reproducibility was investigated by analyzing a spiked serum sample (containing 10 nmol/L) five times. The results showed that RSD value was 8.6%,indicating an acceptable level of precision.
Spiked serum samples with different concentrations of 2,4-DNP were assayed to evaluate the analytical applicability of the proposed method. The results indicated that spiked recoveries were changed from 95.8% to 103.4%. To further investigate the practical perspective of the proposed method,eight serum samples were analyzed by this method and the traditional LC-MS/MS method. The results obtained by the proposed method were linearly correlated to those by traditional method (r = 0.9964) (Fig. S3 in Supporting information). Thus,this method provided a promising method for monitoring of 2,4-DNP in serum samples to control the banned slimming drug. 4. Conclusion
A rapid,simple and selective method has been developed for the determination of 2,4-dinitrophenol in serum samples based on molecularly imprinted,spin column extraction coupled with fluorescence detection. It was shown that only 2,4-dinitrophenol remained on the spin column without the interference of structural analogs. In a word,this method can be used for monitoring of 2,4- DNP in serum samples to control the banned slimming drug. Acknowledgment
This work was supported by National Key Technology R&D Program in the 11th Five-Year Plan of China (No. 2009BADB9B02) Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.06.015.
| [1] | L. Phillips, M.A. Singer, Peripheral neuropathy due to dinitrophenol used for weight loss: something old, something new, Neurology 80 (2013) 773-774. |
| [2] | J. Jiang, Z. Yuan, W. Huang, J. Wang, 2,4-Dinitrophenol poisoning caused by nonoral exposure, Toxicol. Ind. Health 27 (2011) 323-327. |
| [3] | J. Grundlingh, P.I. Dargan, M. El-Zanfaly, D.M. Wood, 2,4-Dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death, J. Med. Toxicol. 7 (2011) 205-212. |
| [4] | R. Loos, S. Tavazzi, B. Paracchini, E. Canuti, C. Weissteiner, Analysis of polar organic contaminants in surface water of the northern Adriatic Sea by solid-phase extraction followed by ultrahigh-pressure liquid chromatography-QTRAP(R) MS using a hybrid triple-quadrupole linear ion trap instrument, Anal. Bioanal. Chem. 405 (2013) 5875-5885. |
| [5] | A. Rahimi, P. Hashemi, A. Badiei, P. Arab, A.R. Ghiasvand, CMK-3 nanoporous carbon as a new fiber coating for solid-phase microextraction coupled to gas chromatography-mass spectrometry, Anal. Chim. Acta 695 (2011) 58-62. |
| [6] | Y. Liu, L.H. Zhu, Y.Y. Zhang, H.Q. Tang, Electrochemical sensoring of 2,4-dinitrophenol by using composites of graphene oxide with surface molecular imprinted polymer, Sens. Actuators B: Chem. 171-172 (2012) 1151-1158. |
| [7] | Z.H. Wang, J.F. Xia, Q. Han, et al., Multi-walled carbon nanotube as a solid phase extraction adsorbent for analysis of indole-3-butyric acid and 1-naphthylacetic acid in plant samples, Chin. Chem. Lett. 24 (2013) 588-592. |
| [8] | Z.H. Wang, J.F. Xia, F.Y. Zhao, et al., Determination of benzoic acid in milk by solidphase extraction and ion chromatography with conductivity detection, Chin. Chem. Lett. 24 (2013) 243-245. |
| [9] | A. Namera, A. Takeuchi, T. Saito, et al., Sequential extraction of inorganic arsenic compounds and methyl arsenate in human urine using mixed-mode monolithic silica spin column coupled with gas chromatography-mass spectrometry, J. Sep. Sci. 35 (2012) 2506-2513. |
| [10] | A. Nakamoto, M. Nishida, T. Saito, et al., Monolithic silica spin column extraction and simultaneous derivatization of amphetamines and 3,4-methylenedioxyamphetamines in human urine for gas chromatographic-mass spectrometric detection, Anal. Chim. Acta 661 (2010) 42-46. |
| [11] | Y. Wang, T.X. Wei, Surface plasmon resonance sensor chips for the recognition of bovine serum albumin via electropolymerized molecularly imprinted polymers, Chin. Chem. Lett. 24 (2013) 813-816. |
| [12] | Y.L. Wu, Y.S. Yan, J.M. Pan, et al., Fabrication and evaluation of molecularly imprinted regenerated cellulose composite membranes via atom transfer radical polymerization, Chin. Chem. Lett. 25 (2013) 273-278. |
| [13] | T. Jing, H. Xia, J. Niu, et al., Determination of trace 2,4-dinitrophenol in surface water samples based on hydrophilic molecularly imprinted polymers/nickel fiber electrode, Biosens. Bioelectron. 26 (2011) 4450-4456. |
| [14] | A. Mehdinia, M. Ahmadifar, M.O. Aziz-Zanjani, A. Jabbari, M.S. Hashtroudi, Selective adsorption of 2,4-dinitrophenol on molecularly imprinted nanocomposites of mesoporous silica SBA-15/polyaniline, Analyst 137 (2012) 4368-4374. |
| [15] | A. Mehdinia, K.T. Baradaran, A. Jabbari, M.O. Aziz-Zanjani, E. Ziaei, Magnetic molecularly imprinted nanoparticles based on grafting polymerization for selective detection of 4-nitrophenol in aqueous samples, J. Chromatogr. A 1283 (2013) 82-88. |




