b Nano and Biotechnology Research Group, University of Mazandaran, Babolsar 47416-95447, Iran;
c Department of Microbiology, Faculty of Science, University of Mazandaran, Babolsar 47416-95447, Iran
The isoquinolines are of great importance to humanity because of their biological and medicinal activities [1]. In particular,1,2- dihydroisoquinolines exhibit antidepressant [2],sedative [3], antitumor and antimicrobial activities [4]. Recently,the 1,4- dipolar intermediates generated by reacting isoquinoline with acetylenic esters were trapped by NH-acids such as indoles [5, 6] and amides [7] and CH-acids like phenyl acetylene [8],β-diketones [9, 10, 11] and β-nitro ketones [12] as well as OH-acids,such as 6- hydroxy1-benzofuran [13]. On the other hand,the interest in biological substances involving kojic acid and 8-hydroxyquinoline increased. Kojic acid,8-hydroxyquinoline and their derivatives have shown to possess various bioactivities such as antimicrobial [14, 15, 16],antitumor [17, 18],antioxidant [19],antibacterial [20, 21, 22]. On the basis of these reports,we were encouraged to synthesize novel 1,2-dihydroisoquinolines,including kojic acid or 8-hydroxyquinoline moieties within their structures.
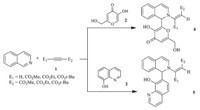
We herein report the reactions of isoquinoline and acetylenic esters 1a-e with kojic acid 2 or 8-hydroxyquinolone 3 as OH-acids to synthesize 1,2-dihydroisoquinolines 4a-e and 5a,5d-e, respectively (Scheme 1).
|
Download:
|
| Scheme 1.Reactions of isoquinoline and acetylenic esters 1 with kojic acid 2 or 8-hydroxyquinolone 3 as OH-acids. | |
Pyridine,isoquinoline,kojic acid,8-hydroxy quinoline and electron-deficient acetylenic esters were obtained from Fluka (Buchs,Switzerland) and Merck (Germany) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. The IR spectra were recorded on a FT-IR Bruker Vector 22 spectrometer. The 1H NMR and 13C NMR spectra were measured with a Bruker DRX-400 AVANCE spectrometer at 400.13 MHz and 100.6 MHz,respectively. Mass spectra were measured on a Finnigan-Matt 8430 mass spectrometer operating at an ionization potential of 30 eV. 2.1. General procedure for synthesis of compound 4
To a mixture of kojic acid (2 mmol) and acetylenic esters (2 mmol) in THF (10 mL),was added isoquinoline (2 mmol). The reaction mixture was stirred for 2 h at room temperature for dialkyl acetylenedicarboxylates or 7-8 h at 60 ℃ for alkyl acetylenecarboxylates. The progress of the reaction was monitored by TLC. After completion of the reaction,the solvent was removed under reduced pressure,and the residue was purified by column chromatography (SiO2; hexane:ethyl acetate = 1:4,v:v) the products 4a-e were obtained in good to high yields (58%-80%) as white or yellow powders.
Dimethyl (2Z)-2-[1-[3-hydroxy-6-(hydroxymethyl)-4-oxo-4Hpyran- 2-yl]isoquinolin-2(1H)-yl]but-2-enedioate (4a): Yellow powder,yield: 80% mp 112-114 ℃. IR (KBr,cm-1): υmax 3422 (OH),1739 and 1701 (2C=O). 1H NMR (400.1 MHz,DMSO-d6): δ 3.56 (s,3H,OCH3),3.85 (s,3H,OCH3),4.09 and 4.13 (AB quartet and doublet,2H,2JHH = 16.0 Hz,3JHH = 6.4 Hz,OCH2),5.24 (s,1H,N- C=CH),5.60 (t,1H,3JHH = 6.4 Hz,OH),5.95 (d,1H,3JHH = 8.0 Hz, =CH),6.26 (s,1H,NCH),6.43 (s,1H,CH),6.50 (d,1H,3JHH = 8.0 Hz, =CH),7.17 (d,1H,3JHH = 7.2 Hz,CHaromatic),7.20-7.28 (m,3H, 3CHaromatic),10.06 (s,1H,OH). 13C NMR (100.6 MHz,DMSO-d6): δ 51.5 and 53.7 (2OCH3),53.8 (NCH),59.8 (OCH2),90.4 (CH),107.8 (CH),109.6 (O-C=CH),125.4,126.6,127.3 and 128.1 (4CH),128.6 (Cq),129.3 (CH),129.6,139.7,147.2,149.6 and 164.6 (5Cq),166.6 and 168.4 (2C=O,esters),173.8 (C=O,pyrone). MS (m/z %): 413 (M+,5),382 (M+-OCH3,2),354 (M+-CO2Me,26),322 [M+-(CO2Me+OMe+H),5],272 (M+-C6H5O4,12),252 [M+-(2OMe+C4H3O3),13],225 [M++1-(CO2Me+OMe+C4H3O3),24],167 (C7H5NO4+,9),129 (C9H7N+,100),102 (C8H6+,26). 2.2. General procedure for synthesis of compound 5
To a mixture of 8-hydroxy quinoline (2 mmol) and acetylenic esters (2 mmol) in dichloromethane (10 mL),was added isoquinoline (2 mmol). The reaction mixture was stirred for 30 min at room temperature for dialkyl acetylenedicarboxylates or 1 h at room temperature for alkyl acetylenecarboxylate. The progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was removed under reduced pressure. The solid residue was purified and washed further with diethyl ether and products 5a and 5d-e were obtained in high yields (62%-94%) as yellow powders. 2.3. General procedure for evaluation of antibacterial activity
In vitro antibacterial activity of the compounds 4a,4d-e,5a and 5d-e was assayed using the disc diffusion method with determination of inhibition zones [36]. The Gram negative and Gram positive test organisms were used as follows: Escherichia coli PTCC 1330,Pseudomonas aeruginosa PTCC 1074,Staphylococcus aureus ATCC 35923 and Bacillus subtilis PTCC 1023 [37]. The late exponential phases of the bacteria were standardized with a final cell density of approximately 108 cfu/mL. Muller-Hinton agar (Merck) were prepared and inoculated from the standardized cultures of the test organisms,then spread as uniformly as possible throughout the entire media. Sterile paper discs (6 mm diameter, Padtan,Iran) were impregnated with 20 μL of the compound solution (20 mg/mL in DMSO) then placed on the upper layer of the seeded agar plate and incubated at 37 ℃ for 24 h. The antibacterial activities of the compounds 4a,4d-e,5a and 5d-e were compared with known antibiotic gentamicin (10 μg/disc) and chloramphenicol (30 μg/disc) as positive control and DMSO (20 μL/disc) as negative control. Antibacterial activity was evaluated by measuring the diameter of inhibition zone (mm) on the surface of plates and the results reported as mean ± SD after three repeats. 3. Results and discussion 3.1. Chemistry
The reaction of kojic acid and dimethyl acetylenedicarboxylate (DMAD) with isoquinoline at room temperature afforded compound 4a in good yield (80%). Similarly,8-hydroxyquinoline reacted smoothly with dimethyl acetylenedicarboxylate (DMAD) and isoquinoline to give the desired 1,2-dihydroisoquinoline 5a in high yield (95%). In order to examine the scope and limitations of this reaction,we extended our study to the other acetylenic diesters and monoesters and the results are shown in Table 1. When the reaction was performed with alkyl acetylenecarboxylate, instead of dialkyl acetylenedicarboxylate,with only one electron-withdrawing substituent (CO2R),the yield of the corresponding product was decreased (Table 1,comparing entries 1 with 4,entries 6 with 7 and 2 with 5). This might probably be due to the lower electrophilicity of the β-carbon of monoesters. Interestingly,sterically hindered diesters,such as di-tert-butyl acetylenedicarboxylate reacted with kojic acid to produce the corresponding 1,2-dihydroisoquinoline 4c in good yield (73%).
| Table 1 Reaction of isoquinoline,kojic acid,8-hydroxyquinoline and acetylenic esters. |
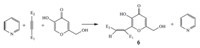
It is noteworthy to mention that when pyridine was used instead of isoquinoline in reactions with kojic acid in the presence of acetylenic esters under the same reaction conditions,the corresponding C-vinylated products of kojic acid were obtained as reported previously (Scheme 2) [23].
|
Download:
|
| Scheme 2.Reactions of pyridine with kojic acid in the presence of acetylenic esters. | |
When quinoline was used in this reaction,in all cases a complex mixture was obtained,which could not be identified.
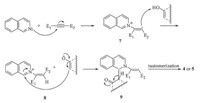
A proposed mechanism for the reactions is shown in Scheme 3. On the basis of the well-established chemistry of N-heterocyclic nucleophiles [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35],it is reasonable to assume that the 1:1 zwitterionic intermediate 7 results from an initial addition of the isoquinoline to acetylenic ester. This intermediate is protonated by the OH-acid which leads to the cationic intermediate 8. The conjugate base of the OH-acid attacks at position 1 of the isoquinolinium ring by the negative carbon atom of the enolate moiety to produce intermediate 9 that tautomerizes to compound 4 or 5 (Scheme 3).
|
Download:
|
| Scheme 3.A proposed mechanism for the reactions of isoquinoline and acetylenic esters 1 with kojic acid 2 or 8-hydroxyquinolone 3 as OH-acids. | |
The structures of 4a-e,5a and 5d-e were deduced from their IR, 1H NMR,13C NMR spectra and from their mass spectrometric data. The 1H NMR spectrum of 4a showed two singlets at 3.56 and 3.85 ppm for the two methoxy groups,an AB quartet and doublet at 4.09 and 4.13 ppm (2JHH = 16.0 Hz,3JHH = 6.4 Hz) for the methylene group (CH2OH),a singlet at 5.24 ppm for the methine group (N-C=CH),a triplet at 5.60 ppm (3JHH = 6.4 Hz) for the OH group of CH2OH,two doublets at 5.95 and 6.50 ppm (3JHH = 8.0 Hz) for the two CH groups of heterocyclic moiety,a singlet at 6.26 ppm for NCH group,a singlet at 6.43 ppm for the CH group of the kojic acid moiety,a multiplet at 7.17-7.28 ppm for the four aromatic protons and a singlet at 10.06 ppm for the enolic proton. The 13C NMR spectrum of 4a exhibited twenty one signals in agreement with the proposed structure. The IR spectrum of 4a showed a broad signal at 3422 cm-1 for the OH groups and two strong signals at 1739 and 1701 cm-1 for the carbonyl groups. The mass spectrum of this compound displayed a molecular ion peak at m/z 413 (5%). Initial fragmentations involved loss of the side chains of the 1,2- dihydroisoquinoline moiety. The 1H NMR and 13C NMR spectra of 4b-e were similar to those of 4b,except for their ester groups, which displayed characteristic resonances in the appropriate regions of the spectra.
The 1H NMR spectrum of 5a showed two singlets at 3.61 and 3.98 ppm for the two methoxy groups,a singlet at 5.46 ppm for the CH group (N-C=CH),two doublets at 5.95 and 6.57 ppm (3JHH = 8.0 Hz) for the two CH groups of heterocyclic moiety,a singlet at 6.74 ppm for the NCH group,a multiplet at 7.07- 8.81 ppm for the nine aromatic protons and a singlet at 8.96 ppm for the OH group. The 13C NMR spectrum of 5a exhibited twentyfour signals in agreement with the proposed structure. The IR spectrum of 5a showed a broad signal at 3280 cm-1 for the OH groups and two strong signals at 1742 and 1708 cm-1 for the carbonyl groups. The mass spectrum of this compound displayed a molecular ion peak at m/z 418 (21%). Initial fragmentations involved loss of the side chains of the 1,2-dihydroisoquinoline moiety.
The 1H NMR and 13C NMR spectra of compounds 5d-e were similar to 5a,except for their ester groups. 3.2. Antibacterial activity
The in vitro antibacterial activity of the compounds 4a,4d-e,5a and 5d-e was evaluated against Gram negative E. coli and P. aeruginosa and Gram positive S. aureus and B. subtilis. The finding towards inhibition of microorganisms was correlated with the standard antibiotics gentamicin and chloramphenicol (Table 2). The results indicated that the compounds 4a,4d and 4e have moderate to good growth inhibitory effects against some microorganisms and among the bacteria tested,compounds 4a,4d and 4e exhibited their highest antibacterial activity against B. subtilis. The values obtained for activity for compound 4a represents good activity against all test bacteria,whereas the growth inhibition of compound 4e showed low activity against P. aeruginosa,but 5a and 5d-e were only effective against Gram positive bacteria (Table 2).
| Table 2 Antibacterial activity of the compounds 4a,4d-e,5a and 5d-e using Kirby-Bauer disc diffusion method. |
In conclusion,we have reported an efficient approach for the synthesis of 1,2-dihydroisoquinolines using isoquinoline and activated acetylenes in the presence of kojic acid or 8-hydroxy quinolone as OH-acids. The simplicity of the present procedure, including mild reaction conditions and high yields of products, makes it an interesting process to the other approaches. The observed antibacterial properties suggest that all compounds exhibited moderate to good antibacterial activity and can be further developed for application as effective antimicrobial agents.
| [1] | (a) C.P. Hansch, G. Sammes, J.B. Taylor, Comprehensive Medicinal Chemistry, Pergamon Press, Oxford, 1990; (b) K.W. Bentley, The Isoquinoline Alkaloide, Pergamon Press, London, 1965; (c) K.W. Bentley, b-Phenylethylamines and the isoquinoline alkaloids, Nat. Prob. Rep. 18 (2001) 148-170; (d) J.D. Scott, R.M. Williams, Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics, Chem. Rev. 102 (2002) 1669-1730. |
| [2] | (a) B.E. Maryanoff, D.F. Mc Comsey, J.F. Gardocki, et al., Pyrroloisoquinoline antidepressants. 2. In-depth exploration of structure-activity relationships, J. Med. Chem. 30 (1987) 1433-1454; (b) K.L. Sorgi, C.A. Maryanoff, D.F. Mc Comsey, D.W. Graden, B.E. Maryanoff, Asymmetric induction in an enammonium-iminium rearrangement. Mechanistic insight via NMR, deuterium labeling, and reaction rate studies. Application to the stereoselective synthesis of pyrroloisoquinoline antidepressants, J. Am. Chem. Soc. 112 (1990) 3567-3579. |
| [3] | E. Lukevics, I. Segal, A. Zablotskaya, S. Germane, Synthesis and neurotropic activity of novel quinoline derivatives, Molecules 2 (1997) 180-185. |
| [4] | (a) L.F. Tietze, N. Rackehmann, I. Miller, Enantioselective total syntheses of the ipecacuanha alkaloid emetine, the alangium alkaloid aubulosine and a novel benzoquinolizidine alkaloid by using a domino process, Chem. Eur. J. 10 (2004) 2722-2731; (b) H.J. Knjlker, S. Agarwal, Total synthesis of the antitumor active pyrrolo[2,1- a]isoquinoline alkaloid (±)-crispine A, Tetrahedron Lett. 46 (2005) 1173-1175. |
| [5] | J.S. Yadav, B.V. Subba Reddy, N.N. Yadav, M.K. Gupta, Three-component coupling reactions of isoquinolines, dimethyl acetylenedicarboxylate and indoles: a facile synthesis of 3-indolyl-1,2-dihydro-2-isoquinolinyl-2-butenedioate, Tetrahedron Lett. 49 (2008) 2815-2819. |
| [6] | M. Nassiri, M.T. Maghsoodlou, R. Heydari, S.M. Habibi Khorassani, Novel multicomponent reactions involving isoquinoline or phenanthridine and activated acetylenic ester in the presence of heterocyclic NH or 1,3-dicarbonyl compounds, Mol. Divers. 12 (2008) 111-117. |
| [7] | I. Yavari, M. Ghazanfarpour-Darjani, M. Sabbaghan, Z. Hossaini, Synthesis of dimethyl 1,2-dihydroisoquinolines through the reaction of isoquinoline and dimethyl acetylenedicarboxylate in the presence of amides, Tetrahedron Lett. 48 (2007) 3749-3751. |
| [8] | J.S. Yadav, B.V. Subba Reddy, N.N. Yadav, M.K. Gupta, B. Sridhar, Gold(Ⅲ) chloridecatalyzed three-component reaction: a facile synthesis of alkynyl derivatives of 1,2-dihydroquinolines and isoquinolines, J. Org. Chem. 73 (2008) 6857-6859. |
| [9] | A. Shaabani, A.H. Rezayan, A. Sarvary, M. Heidary, N. Seik Weng, Synthesis of highly stable unusual charge separated pyridinium, isoquinolinium, quinolinium, and Nmethylimidazolium tetronic acid zwitterions, Tetrahedron 65 (2009) 6063-6068. |
| [10] | E.Y. Xia, J. Sun, R. Yao, C.G. Yan, Synthesis of zwitterionic salts via three component reactions of nitrogen-containing heterocycles, acetylenedicarboxylate and cyclic 1,3-dicarbonyl compounds, Tetrahedron 66 (2010) 3569-3574. |
| [11] | M. Anary-Abbasinejad, H. Anaraki-Ardakani, M.H. Mosslemina, H.R. Khavasi, Isoquinoline-catalyzed reaction between 4-hydroxycoumarin or 4-hydroxy-6- methylpyran-1-one and dialkyl acetylene dicarboxylates: synthesis of coumarin and pyranopyrane derivative, J. Braz. Chem. Soc. 21 (2010) 319-323. |
| [12] | I. Yavari, M. Piltan, L. Moradi, Synthesis of pyrrolo[2,1-a]isoquinolines from activated acetylenes, benzoylnitromethanes, and isoquinoline, Tetrahedron 65 (2009) 2067-2071. |
| [13] | F. Khaleghi, L.B. Din, I. Jantan, W.A. Yaacob, M.A. Khalilzadeh, Facile synthesis of novel 1,4-benzoxazepin-2-one derivatives, Tetrahedron Lett.52 (2011) 7182-7184. |
| [14] | J. Brtko, L. Rondahl, M. Fickova, et al., Kojic acid and its derivatives: history and present state of art, Cent. Eur. J. Public Health 12 (2004) S16-S20. |
| [15] | R. Bentley, From miso, saké and shoyu to cosmetics: a century of science for kojic acid, Nat. Prod. Rep. 23 (2006) 1046-1062. |
| [16] | A.Y. Shen, C.P. Chen, S.A. Roffler, Chelating agent possessing cytotoxicity and antimicrobial activity: 7-morpholinomethyl-8-hydroxyquinoline, Life Sci. 64 (1999) 813-825. |
| [17] | Y. Higa, M. Kawawbe, K. Nabae, et al., Kojic acid-absence of tumor-initiating activity in rat liver, and of carcinogenic and photo-genotoxic potential in mouse skin, J. Toxicol. Sci. 32 (2007) 143-159. |
| [18] | P. Collery, F. Lechenault, A. Cazabat, et al., Inhibitory effects of gallium chloride and tris (8-quinolinolato) gallium Ⅲ on A549 human malignant cell line, Anticancer Res. 20 (2000) 955-958. |
| [19] | F.C. Wehner, P.G. Thiel, S.J. Van Rensburg, I.P.C. Demasius, Mutagenicity to Salmonella typhimurium of some Aspergillus and Penicillium mycotoxins, Mutat. Res. 58 (1978) 193-203. |
| [20] | D. Hudecova, M. Uher, J. Brtko, Halogenderivatives of kojic acid with antifungal effects, Biologia (Bratislava) 47 (1992) 483-488. |
| [21] | A. Albert, S.D. Rubbo, R.J. Goldacre, B.G. Balfour, The influence of chemical constitution of antibacterial activity. A study of 8-hydroxyquinolin (oxine) and related compounds, Br. J. Exp. Pathol. XXVⅢ (1947) 69-87. |
| [22] | El-R. Kenawy, Biologically active polymers IV. Synthesis and antimicrobial activity of polymers containing 8-hydroxyquinoline moiety, J. Appl. Polym. Sci. 82 (2001) 1364-1374. |
| [23] | S. Asghari, M. Faraji-Najjarkolaee, M. Ahmadipour, Regioselective vinylation of kojic acid using acetylenic esters in the presence of triphenylphosphine or tertbutyl isocyanide, Monatsh. Chem. 141 (2010) 781-786. |
| [24] | S. Asghari, A. Khabbazi Habibi, One pot three-component regioselective and diastereoselective synthesis of halogenated pyrido[2,1-b][1,3]oxazines, Tetrahedron 68 (2012) 8890-8898. |
| [25] | R. Huisgen, M. Morikawa, K. Herbig, E. Brunn, 1.4-Dipolare cycloadditionen, Ⅱ. Dreikomponenten-reaktionen des isochinolins mit acetylendicarbonsäureester und verschiedenen dipolarophilen, Chem. Ber. 100 (1967) 1094-1106. |
| [26] | V. Nair, S. Devipriya, S. Eringathodi, Efficient synthesis of [1,3]oxazino[2,3- a]quinoline derivatives by a novel 1,4-dipolar cycloaddition involving a quinoline- DMAD zwitterion and carbonyl compounds, Tetrahedron Lett. 48 (2007) 3667-3670. |
| [27] | A.N. Pillai, B. Rema Devi, E. Suresh, V. Nair, An efficient multicomponent protocol for the stereoselective synthesis of oxazinobenzothiazole derivatives, Tetrahedron Lett. 48 (2007) 4391-4393. |
| [28] | V. Nair, S. Devipriya, E. Suresh, Construction of heterocycles via 1,4-dipolar cycloaddition of quinoline-DMAD zwitterion with various dipolarophiles, Tetrahedron 64 (2008) 3567-3577. |
| [29] | M. Adib, E. Sheibani, M. Mostofi, K. Ghanbary, H.R. Bijanzadeh, Efficient highly diastereoselective synthesis of 1,8a-dihydro-7H-imidazo[2,1-b][1,3]oxazines, Tetrahedron 62 (2006) 3435-3438. |
| [30] | I. Yavari, A. Mirzaei, Z. Hossaini, S. Souri, Diastereoselective synthesis of fused[1,3]oxazines from ethyl pyruvate, activated acetylenes and N-heterocycles, Mol. Divers. 14 (2010) 343-347. |
| [31] | I. Yavari, Z. Hossaini, S. Souri, S. Seyfi, Diastereoselective synthesis of fused[1,3]thiazolo[1,3]oxazins and [1,3]oxazino[2,3-b][1,3]benzothiazoles, Mol. Divers. 13 (2009) 439-443. |
| [32] | I. Yavari, Z. Hossaini, M. Sabbaghan, M. Ghazanfarpour-Darjani, Reaction of Nheterocycles with acetylenedicarboxylates in the presence of N-alkylisatins or ninhydrin. Efficient synthesis of spiro compounds, Monatsh. Chem. 138 (2007) 677-681. |
| [33] | I. Yavari, N. Hosseini, L. Moradi, An efficient synthesis of 2-cyano-2-phenyl-2, 11b-dihydro-[1,3]oxazino[2,3-a]isoquinolines by reaction of isoquinoline with electron-deficient acetylenes in the presence of benzoylcyanide, Monatsh. Chem. 139 (2008) 953-956. |
| [34] | M.B. Teimouri, T. Abbasi, S. Ahmadian, M.R. Poor Heravi, R. Bazhrang, An efficient three-component protocol for the synthesis of novel spiro-oxazinobarbiturates, Tetrahedron 65 (2009) 8120-8124. |
| [35] | A.A. Esmaeili, H. Vesalipoor, R. Hosseinabadi, et al., An efficient diastereoselective synthesis of spiro pyrido[2,1-b][1,3]oxazines via a novel pyridine-based threecomponent reaction, Tetrahedron Lett. 52 (2011) 4865-4867. |
| [36] | M. Mohseni, H. Norouzi, J. Hamedi, A. Roohi, Screening of antibacterial producing actinomycetes from sediments of the Caspian Sea, Int. J. Mol. Cell Med. 2 (2013) 64-71. |
| [37] | S. Asghari, S. Ramezani, M. Mohseni, Synthesis and antibacterial activity of ethyl 2-amino-6-methyl-5-oxo-4-aryl-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carboxylate, Chin. Chem. Lett. 25 (2014) 431-434. |