During the past ten years significant advances have been achieved in the development of C-N cross-coupling reactions [1, 2, 3, 4]. In the presence of several transition metal catalysts (Cu,Pd,Ni, etc.),various nitrogen-containing compounds,such as pharmaceuticals,agrochemicals and other functional molecules,could be efficiently prepared. In terms of the cost and toxicity,the coppercatalyzed Ullmann-type C-N coupling reaction represents one of the most desirable methods to prepare the numerousN-containing molecules in chemical industry [2]. The pioneering work has established the fact that ligands are crucial in the copper-catalyzed C-N coupling reactions [1].
The indole-containing compounds are among the most important and ubiquitous heterocyclic frameworks in nature. CoppercatalyzedN-arylation of nitrogen heterocycles with aryl halides have been developed with various ligands. In 2002,Buchwald first found that the diamine ligands were efficient for copper catalyzed N-arylation of simple indoles [5, 6, 7]. In 2004,Taillefer developed Chxn-Py-Al-copper complex for the C-N coupling reactions [8]. Subsequently,many useful ligands,such as L-proline [9],1,1'-binaphthyl-2,2'-diamine [10],benzotriazole [11],2-(2'-pyridyl)-benzimidazole [12],8-hydroxyquinalidine [13],tetrazole-1-acetic acid [14] and 4,7-dipyrrolidinyl-1,10-phenanthroline [15],were developed for promoting the copper-catalyzed N-arylation of indoles and other nitrogen heterocycles with aryl halides. Recently, we have been focusing on the preparation ofN-containing heterocycles based on cheap and green catalyst systems (Cu-based catalysts)viaC-N cross-coupling reactions (Scheme 1c). Other elegant catalytic [16, 17, 18, 19, 20, 21] or non-catalytic [22, 23, 24, 25] methods were developed for the facile synthesis of substituted indoles. Recently,Lutz Ackermann and coworkers [26, 27] developed a one-pot substituted indole synthesis base on Pd/Cu catalysis (Scheme 1a).Katz and coworkers [28] reported an interesting strong base mediated benzodipyrrole syntheses from diethynyldifluorobenzenes (Scheme 1b). To the best of our knowledge,there are limited examples for theN-arylation of 2-arylindoles and its derivatives viaCu-catalysis. Herein,we reported our results on the coppercatalyzed N-arylation of arylindoles with aryl halides in the presence of the DMEDA ligand with excellent yields under mild conditions.

|
Download:
|
| Scheme 1. Methods for the preparation of 1,2-arylindoles. | |
Reagents were obtained commercially and used as received. Solvents were purified and dried by standard methods. Toluene was dried and distilled from sodium/benzophenone immediately prior to use under a nitrogen atmosphere. Unless noted otherwise, all other compounds have been reported in the literatures or are commercially available. All reactions were performed in ovendried glassware. Thin layer chromatography (TLC) employed glass 0.25 mm silica gel plates. Flash chromatography columns were packed with 200-300 mesh silica gel. 1H NMR and 13C NMR data were recorded in CDCl3solutions with Varian Mercury (300 MHz) spectrometers using tetramethylsilane (TMS) as an internal standard. Analytical gas chromatography (GC) was performed using an Aglient 6890 Gas Chromatography fitted with a flame ionization detector.
Representative procedure for the synthesis of 1,2-diphenylindole ( 3a ): A 10 mL Schlenk tube was charged with 2-phenylindole 1a (193.2 mg,1.0 mmol),CuI (10 mol%,19.0 mg,0.1 mmol) and K3PO4(424 mg,2.0 mmol). The Schlenk tube was evacuated and filled with N2 (this procedure was repeated three times), and then toluene (2.0 mL),DMEDA (20 mol%,17.6 mg,0.2 mmol) and iodobenzene 2a (244.8 mg,1.2 mmol) were added. The resulting mixture was stirred at 110°C for 24 h. After cooling to room temperature,the reaction mixture was quenched and extracted with ethyl acetate (10 mL×3). The organic extracts were combined,dried over Na2SO4 and concentrated under reduced pressure,and then purified by silica gel chromatograph (petroleum ether) to yield the desired product as a white solid (245.1 mg,91% yield). The data of compounds 3a -qcan be found in Supporting information. 3. Results and discussion
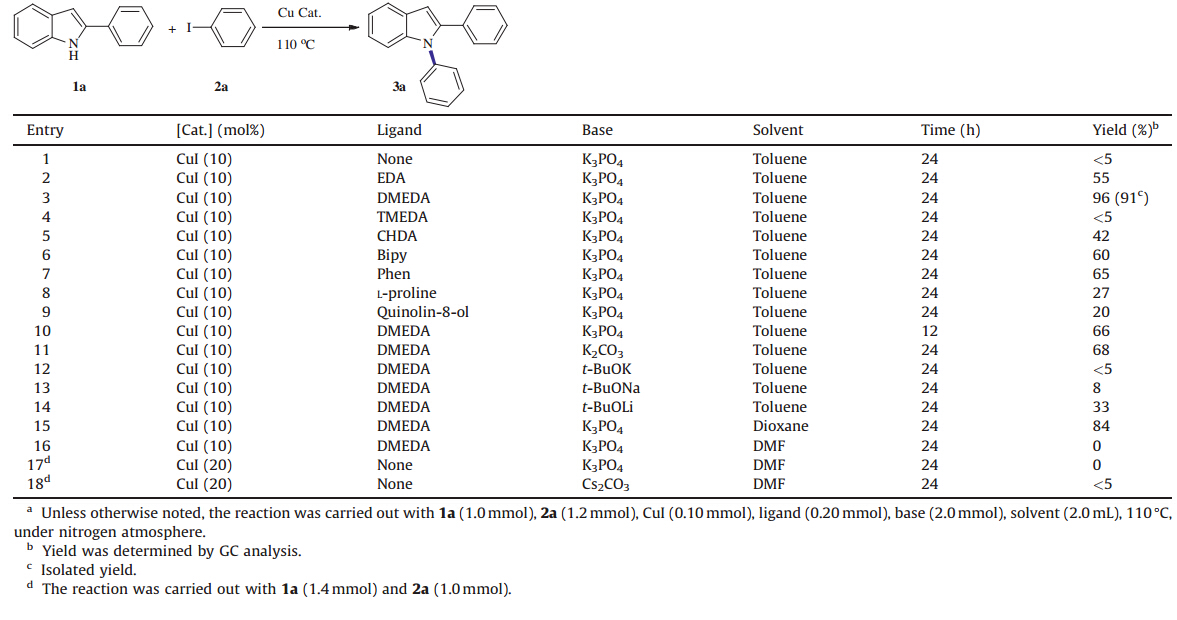
The C-N coupling reaction between 2-phenylindole (1a) and iodobenzene ( 2a ) was selected as the model reaction to explore the suitable reaction conditions (Table 1). Commonly used and airstable copper salt CuI was chosen as the catalyst and several other reaction parameters,such as ligand,base and solvent were carefully optimized. Using anhydrous K3PO4(2 equiv.) as the base and toluene as the solvent,a series of bidentateN-containing ligands were tested. In the absence of any ligands,CuI (10 mol%) could not independently promote the conversion of 2-phenylindole (1a) after 24 h at 110°C (Table 1,entry 1). The use of ethylenediamine (EDA) led to a 55% yield of the desiredN-arylation product ( 3a ) (Table 1,entry 2). WhenN,N'-dimethylethylenediamine (DMEDA) was used,a 100% conversion of 1a was observed and a 96% yield of the desired N-arylation product ( 3a ) was obtained (Table 1,entry 3). However,the use of tetramethyl substituted ethylenediamine (TMEDA) resulted in no consumption of 1a (Table 1,entry 4). Presumably,the increased level of substitution at nitrogen leads to reduced binding capacity. Other commonly used ligands,such as cyclohexane-1,2-diamine (CHDA), 2,2'-bipyridine (bipy),1,10-phenanthroline (phen) and L-proline were also examined but all offered poor to moderate yields of 3a (Table 1,entries 5-8). 8-Hydroxyquinoline (quinolin-8-ol) showed inferior efficiency as well (Table 1,entry 9). The preliminary results showed that the combination of air stable CuI (10 mol%) and DMEDA (20 mol%) appeared to be an efficient catalyst system for theN-arylation of 2-phenylindole (1a) with aryl iodides (2). Base is also crucial for this transformation. K3PO4was the most efficient among the tested bases (Table 1,entries 11-14),and other tested strong bases (t-BuOK,t-BuONa andt-BuOLi) produced no or lower conversion (Table 1,entries 12-14). Amongst all tested solvents, toluene is a superior solvent to others (dioxane and DMF) (Table 1, entries 15 and 16). It required 24 h to complete conversion of 1a , while a shorter reaction time (12 h) led to a 66% yield of 3a (Table 1, entry 10). Moreover,ligandless reaction conditions [16, 17] were also examined for this reaction (Table 1,entries 17 and 18),the results showed that using 20 mol% CuI as catalyst and DMF as solvent,no reaction was observed in the presence of K3PO4 or Cs2CO3.
| Table 1 Reaction conditions for C-N coupling.a |
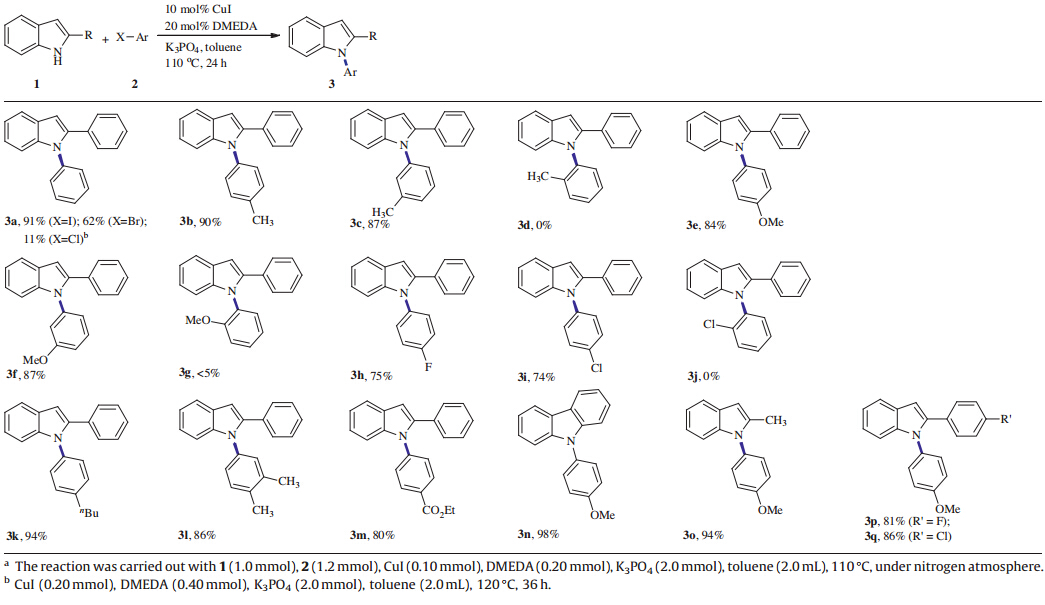
With the optimized reaction conditions in hand,the substrate scope of theN-arylation of 2-arylindole (1) with aryl halides (2) was studied and the results were shown in Table 2. Electron-rich and electron-deficient aryl iodides reacted smoothly with 2-phenylindole (1a) and offered good to excellent yields in most cases. 1-Iodo-4-methylbenzene (2b) and 1-iodo-4-methylbenzene (2c) reacted with 2-phenylindole (1a) to generate the corresponding N-arylation products (3b and 3c) in 90% and 87% yield, respectively. 1-Iodo-4-methoxybenzene (2e) and 1-iodo-3-methoxybenzene (2f) reacted well with 2-phenylindole (1a) and produced the correspondingN-arylation products (3e and 3f)in 84% and 87% yield,respectively. Notably,aryl iodides bearing ortho-substituents (Me,OMe and Cl) reacted sluggishly and no or lower conversions were observed (3d,3g and 3j),which might be attributable to the influence of steric hindrance. The reactions of 1-fluoro-4-iodobenzene and 1-chloro-4-iodobenzene gave 75% and 74% yield (3h and 3i),respectively. Electron-deficient aryl iodide bearing ester group was also favored in this system and produced the corresponding N-arylation product (3m)in 80% yield. Other aryl iodides,such as 1-butyl-4-iodobenzene and 4-iodo-1,2-dimethylbenzene can be efficiently coupled with 2-phenylindole (1a) and gave the coupling products in 94% and 86% yield (3k and 3l),respectively. Under the standard reaction conditions,the Cu-catalyzed N-arylation of other substituted indoles were examined as well. Carbazole and 2-methylindole can undergo the C-N coupling smoothly and afforded the correspondingN-arylation products (3n and 3o) in 98% and 94% yield,respectively. Moreover,2-(4-fluorophenyl)-indole and 2-(4-chlorophenyl)-indole were also suitable under the same reaction conditions and afforded the N-arylation products (3p and 3q) in 81% and 86% yield,respectively.
Aryl bromides were tested under the standard reaction conditions (10 mol% CuI/20 mol% DMEDA) and a 62% yield of the coupled product was obtained after 24 h. When the less reactive Ph-Cl was tested,lower conversion was observed with 20 mol% CuI catalyst at elevated temperature (120°C) after 36 h (Table 2, 3a ). Considering the modest outcome of the less reactive aryl bromides,20 mol% CuI and 40 mol% DMEDA was employed to promote the reactions and good yields (80%-84%) of the correspondingN-arylation products ( 3a ,3b and 3e) were achieved (Scheme 2).
| Table 2 Substrates scope forN-arylation of 2-arylindole.a |

|
Download:
|
| Scheme 2. N-Arylation of 2-phenylindole with aryl bromides. | |
In conclusion,we have developed a simple and efficient method for the synthesis of substituted indoles using cheap CuI as the catalyst and DMEDA as the ligand. Electron-rich and electrondeficient aryl iodides and aryl bromides all can afford the corresponding N-arylation products with 2-arylindole in good to excellent yields under mild reaction conditions. Further investigation on promoting the conversion of aryl halides bearing ortho-substituents will be reported in due course. Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21102036) and Plan for Scientific Innovation Talent of Henan University of Technology (No. 11CXRC02) and startup fund from HAUT (No. 2010BS042). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.04.021.
| [1] | S.V. Ley, A.W. Thomas, Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation, Angew. Chem. Int. Ed. Engl. 42 (2003) 5400-5449. |
| [2] | F. Monnier, M. Taillefer, Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions: copper makes a difference, Angew. Chem. Int. Ed. Engl. 47 (2008) 3096-3099. |
| [3] | Y. Wang, J. Zeng, X. Cui, Recent progress in copper-catalyzed C-N coupling reactions, Chin. J. Org. Chem. 30 (2010) 181-199. |
| [4] | J.D. Senra, L.C.S. Aguiar, A.B.C. Simas, Recent progress in transition-metal catalyzed C-N cross-couplings: emerging approaches towards sustainability, Curr. Org. Synth. 8 (2011) 53-78. |
| [5] | A. Klapars, J.C. Antilla, X. Huang, S.L. Buchwald, A general and efficient copper catalyst for the amidation of aryl halides and the N-arylation of nitrogen heterocycles, J. Am. Chem. Soc. 123 (2001) 7727-7729. |
| [6] | J.C. Antilla, A. Klapars, S.L. Buchwald, The copper-catalyzed N-arylation of indoles, J. Am. Chem. Soc. 124 (2002) 11684-11688. |
| [7] | A. Klapars, X. Huang, S.L. Buchwald, A general and efficient copper catalyst for the amidation of aryl halides, J. Am. Chem. Soc. 124 (2002) 7421-7428. |
| [8] | H. Cristau, P.P. Cellier, J. Spindler, M. Taillefer, Highly efficient and mild coppercatalyzed N-and C-arylations with aryl bromides and iodides, Chem. Eur. J. 10 (2004) 5607-5622. |
| [9] | X. Diao, Y. Wang, Y. Jiang, D. Ma, Assembly of substituted 1H-benzimidazoles and 1,3-dihydrobenzimidazol-2-ones via CuI/L-proline catalyzed coupling of aqueous ammonia with 2-iodoacetanilides and 2-iodophenylcarbamates, J. Org. Chem. 74 (2009) 7974-7977. |
| [10] | R.K. Rao, A.B. Naidu, E.A. Jaseer, G. Sekar, An efficient, mild, and selective Ullmanntype N-arylation of indoles catalyzed by copper(I) complex, Tetrahedron 65 (2009) 4619-4624. |
| [11] | A.K. Verma, J. Singh, R.C. Larock, Benzotriazole: an efficient ligand for the copper-catalyzed N-arylation of indoles, Tetrahedron 65 (2009) 8434-8439. |
| [12] | S. Haneda, Y. Adachi, M. Hayashi, Copper(I)-2-(20-pyridyl)benzimidazole catalyzed N-arylation of indoles, Tetrahedron 65 (2009) 10459-10462. |
| [13] | X. Yang, H. Xing, Y. Zhang, et al., CuI/8-hydroxyquinalidine promoted N-arylation of indole and azoles, Chin. J. Chem. 30 (2012) 875-880. |
| [14] | F. Wu, P. Liu, X. Ma, J. Xie, B. Dai, Tetrazole-1-acetic acid as a ligand for coppercatalyzed N-arylation of imidazoles with aryl iodides under mild conditions, Chin. Chem. Lett. 24 (2013) 893-896. |
| [15] | J. Engel-Andreasen, B. Shimpukade, T. Ulven, Selective copper catalysed aromatic N-arylation in water, Green Chem. 15 (2013) 336-340. |
| [16] | L.B. Zhu, L. Cheng, Y.X. Zhang, R.G. Xie, J.S. You, Highly efficient copper-catalyzed N-arylation of nitrogen-containing heterocycles with aryl and heteroaryl halides, J. Org. Chem. 72 (2007) 2737-2743. |
| [17] | L.B. Zhu, G.C. Li, L. Luo, et al., Highly functional group tolerance in coppercatalyzed N-arylation of nitrogen-containing heterocycles under mild conditions, J. Org. Chem. 74 (2009) 2200-2202. |
| [18] | N. Panda, A.K. Jena, S. Mohapatra, S.R. Rout, Copper ferrite nanoparticle-mediated N-arylation of heterocycles: a ligand-free reaction, Tetrahedron Lett. 52 (2011) 1924-1927. |
| [19] | N.V. Suramwar, S.R. Thakare, N.N. Karade, N.T. Khaty, Green synthesis of predominant (1 1 1) facet CuO nanoparticles: heterogeneous and recyclable catalyst for N-arylation of indoles, J. Mol. Cat. A: Chem. 359 (2012) 28-34. |
| [20] | F.P. Yi, H.Y. Sun, X.H. Pan, Y. Xu, J.Z. Li, Synthesis of Fischer indole derivatives using carboxyl-functionalized ionic liquid as an efficient and recyclable catalyst, Chin. Chem. Lett. 20 (2009) 275-278. |
| [21] | S.V. Goswami, P.B. Thorat, V.N. Kadam, S.A. Khiste, S.R. Bhusare, A convenient one-pot three component synthesis of 3-aminoalkylated indoles catalyzed by 3-chlorophenylboronic acid, Chin. Chem. Lett. 24 (2013) 422-424. |
| [22] | W.J. Smith III, J.S. Sawyer, A novel and selective method for the N-arylation of indoles mediated by KF/Al2O3, Tetrahedron Lett. 37 (1996) 299-302. |
| [23] | G.L. Frayne, G.M. Green, Investigation of the N-arylation of various substituted indoles using microwave-assisted technology, Tetrahedron Lett. 49 (2008) 7328-7329. |
| [24] | Z.W. Chen, N. Zhang, Z.H. Wang, W.K. Su, An efficient synthesis of novel chromeno[3'4':5,6]pyrano[2,3-b]indole derivatives, Chin. Chem. Lett. 24 (2013) 199-201. |
| [25] | Y.X. Cui, L.D. Sun, Q. Sun, L. Shi, Highly selective synthesis of 3-methylindole from glycerol and aniline over Cu/NaY modified by K22O, Chin. Chem. Lett. 24 (2013) 1127-1129. |
| [26] | L. Ackermann, General and efficient indole syntheses based on catalytic amination reactions, Org. Lett. 7 (2005) 439-442. |
| [27] | L.T. Kaspar, L. Ackermann, Three-component indole synthesis using ortho-dihaloarenes, Tetrahedron 61 (2005) 11311-11316. |
| [28] | N.P. Bizier, J.W. Wackerly, E.D. Braunstein, et al., An alternative role for acetylenes: activation of fluorobenzenes toward nucleophilic aromatic substitution, J. Org. Chem. 78 (2013) 5987-5998. |