b Research Institute of Applied Chemistry, Shanxi University, Taiyuan 030006, China
Chemosensors are devices,molecule-sized or larger,that utilize abiotic receptors to achieve analyte recognition with concomitant irreversible transduction of a human-observable signal [1]. Many sensing methods for detecting Cu2+ have been described,such as colorimetric and fluorescent chemosensors,and electrochemical methods [2, 3, 4, 5, 6, 7, 8, 9, 10, 11]. Colorimetric sensors are promising due to the simplicity of the assay. Furthermore,colorimetric assays have a significantly lower capital cost than the closely related methods, such as fluorescent sensors,for which both spectrophotometric equipments and a UV light source are required [12, 13, 14, 15, 16, 17, 18].
Copper is an essential soft transition metal ion that plays a pivotal role in environmental,biological,and chemical systems [19, 20, 21, 22]. Copper compounds are also employed for plant diseases management,water treatment and as preservatives for wood and leather [23]. Nonetheless,while a low-level background intake of copper is indispensable,high doses of copper can be harmful and even toxic to biological systems [24, 25]. Copper toxicity causes oxidative stress and related symptoms,which may lead to diabetes and many neurodegenerative disorders such as Alzheimer’s [26],Parkinson’s [27],Menke’s [28],and Wilson’s diseases [29]. Owing to the significant physiological relevance and associated biomedical implications,there is considerable interest in developing selective and sensitive copper sensors [30].
Pyridoxal is an ideal platform for the development of colorimetric or fluorescent chemosensors for specific heavy and transition metal ions. We synthesized a pyridoxal-based colorimetric chemosensor,probe (1),for rapid,selective and sensitive response to Cu2+in aqueous media. Solutions of 1 are colorless,but upon addition of micromolar Cu2+a yellow color is obtained. Addition of other common alkali-,alkaline earth-,transition- and rare earth metal ions result in no or minimal spectral change. Compound 1 is a naked-eye chemosensor for the detection of Cu2+upon coordination. At the same time,the fluorescence of the compound1can be quenched by only Cu2+. 2. Experimental 2.1. Materials
All chemicals and solvents were of analytical grade and bought from Sigma-Aldrich or Beijing City without further purification. Chromatography was carried out on silica gel (200-300 mesh). Thin layer chromatography (TLC) was carried out using silica gel GF254 plates with a thickness of 0.20-0.25 mm. Deionized water was used to prepare all aqueous solutions. The solutions of Hg2+, Er3+,Al3+,Ba2+,Cd2+,Zn2+,Cu2+,Mn2+,Ni2+,Co2+,Eu3+,La3+,Sm3+, Fe3+,Gd3+,Nd3+,Ho3+,Sn2+,Yb3+,Ce4+ and Zr3+were prepared from their chloride salts. The solution of Ag+ was prepared from its nitrate salt. The solution of VO2+was prepared from its sulfate salt. All spectroscopic measurements were performed in an HEPES (10 mmol/L,pH 7.4) buffer. HEPES buffer solutions were obtained by adding 1 mol/L NaOH solution into 10 mmol/L aqueous HEPES using a Mettler Toledo pH meter. Probe 1 was dissolved in absolute methanol to prepare the stock solutions with a concentration of 2.0 mmol/L. 2.2. Synthesis of the compound
The synthesis of compounds1is summarized in Scheme 1. 1 was synthesized by a one-step reaction of pyridoxal with excess hydrazine hydrate in ethanol,in accordance with the procedure reported in the literature [31]. An excessive amount of hydrazine hydrate (85%,1.2 mL) was added to a 0.2036 g (1 mmoL) of pyridoxal that had been dissolved in 20 mL of ethanol. The reaction solution was then refluxed for 8 h and a white product was obtained by evacuating ethanol under reduced pressure. The solid product was recrystallized from ethanol-water to give pyridoxal hydrazide as a white powder in 80% yield (0.15 g).
|
Download:
|
| Scheme 1. The synthesis of the probe. | |
The compound was characterized (Fig. S1 in Supporting information): 1H NMR (300 MHz,25°C,DMSO-d6): d 2.36 (s, 3H),4.52 (s,2H),5.28 (s,1H),7.64 (s,2H),7.86 (s,1H),8.18 (s,1H), 12.18 (s,1H); 13C NMR (75 MHz,DMSO-d6):d149.5,143.5,135.0, 130.5,123.2,57.8,16.6; ESI-MS m/z: [Probe + H]+ calcd. for C8H11N3O2 182.09; found 182.42; elemental analysis (calcd. %) for C8H11N3O2: C,52.74; H,6.64; N,23.06; found: C,52.73; H,6.66; N,23.04. 2.4. Physical measurements
The UV-vis spectra were recorded on a Cary 50 Bio UV-visible spectrophotometer in a 4.5 mL (1 cm in diameter) cuvette using 2 mL of solution. Fluorescence spectra were measured on a Cary Eclipse fluorescence spectrophotometer. All data were processed with the Origin 8.0 program. Absorption maxima (lmax) is given in nm. 1H NMR, 13C NMR spectra were recorded on a Bruker AVANCE-300 MHz and 75 MHz NMR spectrometer,respectively. Chemical shifts are given in parts per million downfield from tetramethylsilane (0.0 ppm). ESI-MS was measured with an LC-MS 2010A (Shimadzu) instrument. 2.5. Measurement procedure
The UV-vis procedures were shown as follows: to a 10 mmol/L, pH 7.4 HEPES buffer solution containing 27mmol/L probe1,ion sample was gradually added. All UV-vis spectral data were recorded 30 s after the ion addition.
The fluorescence procedures were as follows: to a 10 mmol/L, pH 7.4 HEPES buffer solution containing 200mmol/L probe1,ion sample was gradually added. All fluorescence spectral data were recorded 30 s after the ion addition. 3. Results and discussion 3.1. Selectivity over metal ions
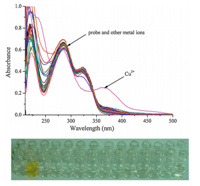
The effect of a wide range of environmentally and physiologically active metal ions on the probe was investigated using the UV- vis spectra of solutions containing the sprobe and the metal ion (100 equiv.) in an HEPES aqueous buffer (10 mmol/L,pH 7.4). The results showed that whereas metal ions such as Hg2+,Zn2+,Ni2+, Bi3+,Cu+ ,Co2+,VO2+,Mn2+,Ru3+,Cd2+,Pb2+,Ag+ ,La3+,Ce4+ ,Yb3+, Cr2+,Er3+,Mg2+,Sn2+,Al3+,Nd3+,Zr4+ ,K+ ,Sm3+,Fe2+,Fe3+and Eu3+do not result in any apparent changes in the relative absorption intensity,there is notable change when Cu2+is involved as shown in Fig. 1.
|
Download:
|
| Fig. 1. UV-vis absorption spectra of probe (27mmol/L) in 50% CH3OH solution (methanol:HEPES = 1:1,v/v,pH 7.4) in the presence of all kinds of analytes (300mmol/L),line one from left to right: Hg2+ ,Zn2+,Ni2+,Bi3+,Cu+ ,Co2+,VO2+,Mn2+, Ru3+,Cd2+,Pb2+,Ag+ ,La3+,Ce4+ ; line two from left to right probe,Cu2+,Yb3+,Cr2+, Er3+,Mg2+,Sn2+,Al3+,Nd3+,Zr4+ ,K+ ,Sm3+,Fe3+and Eu3+. | |
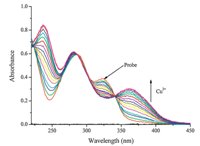
In addition,Fig. 2 shows the change on the UV-vis spectrum when Cu2+was added to the HEPES (10 mmol/L,pH 7.4) solution containing the probe (27mmol/L). As the Cu2+ concentration increased,the absorption peaks at 359 nm gradually decreased. An isosbestic point was noted at 341 nm and a new peak at 359 nm emerged,all of which indicate the formation of a new species. Interestingly,the color of the solution changes from colorless to yellow.
|
Download:
|
| Fig. 2. Absorption spectral changes of probe (27mmol/L) in methanol/HEPES buffer solution (1:1,v/v,10 mmol/L,pH 7.4) upon addition of Cu2+;Cu2+added gradually with [Cu2+] = 0-27mmol/L; each spectrum is recorded 30 s after Cu2+ addition. | |
According to the linear Benesie Hildebrand expression,the measured intensity [1/(A-A0)] at 359 nm varied as a function of 1/[Cu2+] in a linear relationship (R= 0.99315) (Fig. S2 in Supporting information),indicating a 1:1 stoichiometry between Cu2+and the probe. The association constant of 1with Cu2+in HEPES aqueous buffer (10 mmol/L,pH 7.4) was calculated to be 1.99×106L/mol [15, 32, 33, 34]. 3.3. Detection range
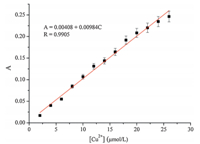
To investigate the detection limit of the probe for Cu2+,the probe (27mmol/L) was treated with various concentrations of Cu2+ (0-27mmol/L) and the relative absorption intensity at 359 nm was plotted as a function of the Cu2+concentration (Fig. 3). The absorption intensity of the probe was linearly proportional to the Cu2+concentrations between 0 and 27mmol/L. The detection limit, based on the definition by IUPAC (CDL= 3 Sb/m) [30, 35, 36, 37],was found to be 0.08mmol/L from 13 blank solutions. The probe shows a high sensitivity toward copper and is comparable to other reported Cu2+chemosensors [38, 39, 40].
|
Download:
|
| Fig. 3. The linear detection range for 0-27mmol/L of Cu2+ at 359 nm. | |
Time-dependency of the probe was monitored in the UV-vis spectra in the presence of 10 equiv. of Cu2+. The kinetic study showed that the reaction was complete within 30 s for Cu2+, indicating that the probe reacts rapidly with Cu2+under the experimental conditions (Fig. S3 in Supporting information). This unprecedented fast response could provide the possibility of quantitative detection without any pretreatment of samples. 3.5. pH dependence
The pH range for the determination of Cu2+was also studied. Fig. S4 in Supporting information showed the absorption intensity obtained for the free probe and probe-Cu2+in different pH values. Fig. S4 showed that the probe had nearly no absorption at 359 nm between pH 5.0 and 11.0 the probe-Cu2+complex had a strong absorption. Meanwhile,when the solution pH value is between 2.0 and 4.0 or in the range of 12-13,there is a strong absorption for both the free probe and probe-Cu2+. Therefore,the pH range of 5.0-11.0 is effective for this probe and a neutral pH was used for further studies. 3.6. Fluorescence spectra titration for Cu2+
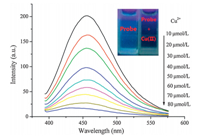
We thus carried out a detailed investigation on the probe recognition of Cu2+. The probe has fluorescence at 455 nm (lex 365 nm). The changes in the fluorescence spectra of the probe (200mmol/L) in the presence of Cu2+ in HEPES buffer (10 mmol/L, pH 7.4) are displayed in Fig. 4. As it can be seen that the addition of Cu2+caused changes in the fluorescence spectra and a remarkable decrease of the emission intensity in the emission maximum was observed.
|
Download:
|
| Fig. 4. Fluorescence spectra of probe (200mmol/L) in the presence of various concentrations of Cu2+(0-80mmol/L) in HEPES (10 mmol/L,pH 7.4) (lex= 365 nm, slit: 5 nm/5 nm). Inset: visual fluorescence changes of probe upon addition of Cu2+in HEPES buffer solution (10 mmol/L,pH 7.4) under illumination with a 365 nmUV lamp. | |
A color change from blue to colorless can be visualized under illumination with a 365 nm UV lamp. 3.7. Proposed mechanism
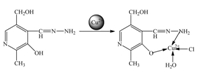
The proposed mechanism of detection and the structures of the probes,both with and without the addition of Cu2+,are shown in Fig. 5. Upon coordinated with Cu2+,the Cu2+can cause absorption of the probe. An isosbestic point was noted at 341 nm and a new peak at 359 nm emerged,all which indicate the formation of a new species. Meanwhile,due to the paramagnetic effect from spin- orbit coupling,Cu2+induces fluorescence quenching.
|
Download:
|
| Fig. 5. The proposed determination mechanism of probe-Cu2+. | |
Mass spectrometry analysis of a product obtained from the reaction of the probe with Cu2+in CH3OH shows binding between the probe and Cu2+. A peak at m/z= 291.92,corresponding to [Probe + CuCl2+H]+ ,is clearly observed (Fig. S5 in Supporting information),which is consistent with a 1:1 stoichiometry between Cu2+and the probe. 3.8. Cellular imaging
The ability of the probe to detect Cu2+within living cells was also evaluated by laser confocal fluorescence imaging using an Olympus FV1000 laser scanning microscope. The optical window at the blue channel (400-500 nm) was chosen as a signal output. As shown in Fig. 6a,under selective excitation at 365 nm, HepG2 cells incubated with 200mmol/L of probe for 30 min at 37°C showed blue fluorescence. In a further experiment it was found that HepG2 cells displayed no fluorescence when the cells were first incubated with 200mmol/L of probe for 30 min at 37°C and then incubated with 80mmol/L of CuCl2 (Fig. 6c).These cell experiments show the good cell-membrane permeability of the probe,and it can thus be used to detect Cu2+within living cells.

|
Download:
|
| Fig. 6. Confocal fluorescence images of HepG2 cells: (a) fluorescence image of HepG2 cells with adding probe (200mmol/L) and its brightfield image (c); (b) fluorescence image of HepG2 cells incubated with 200mmol/L probe for 30 min at 37°C and then incubated with 80mmol/L CuCl2for 30 min at 37°C and its brightfield image (d). | |
A pyridoxal-based derivative was prepared and characterized by 1H NMR, 13C NMR and ESI-MS. The probe exhibited different selectivity at micromolar levels for Cu2+against other ions as measured by UV-vis spectroscopy and displayed a dramatic change in fluorescence intensity. These changes were probably caused by the coordination between the probe and Cu2+and the paramagnetic effect from the spin-orbit coupling of the Cu2+. This probe similarly displayed Cu2+selective chromogenic behavior and turned from colorless to yellow,which allowed naked-eye detection of Cu2+ions in 50% CH3OH aqueous solution. More importantly,an application in bioimaging was illustrated. The probe has good cell-membrane permeability and thus can be used to detect Cu2+within living cells. Acknowledgments
The work was supported by the National Natural Science Foundation of China (No. 21102086),the Shanxi Province Science Foundation for Youths (Nos. 2012021009-4 and 2013011011-1), the Shanxi Province Foundation for Returnee (No. 2012-007),the Taiyuan Technology Star Special (No. 12024703),the Program for the Top Young and Middle-aged Innovative Talents of Higher Learning Institutions of Shanxi (TYMIT,No. 2013802),talents Support Program of Shanxi Province (No. 2014401) and CAS Key Laboratory of Analytical Chemistry for Living Biosystems Open Foundation (No. ACL201304). Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.06.017.
| [1] | V. Dujols, F. Ford, A.W. Czarnik, A long-wavelength fluorescent chemo-dosimeter selective for Cu (II) ion in water, J. Am. Chem. Soc. 119 (1997) 7386-7387. |
| [2] | J. Tan, X.P. Yan, 2,1,3-Benzoxadiazole-based selective chromogenic chemosensor for rapid naked-eye detection of Hg2+ and Cu2+, Talanta 76 (2008) 9-14. |
| [3] | L.P. Singh, J.M. Bhatnagar, Copper(II) selective electrochemical sensor based on Schiff base complexes, Talanta 64 (2004) 313-319. |
| [4] | Y.S. Xie, Y.B. Ding, X. Li, et al., Selective, sensitive and reversible "turn-on" fluorescent cyanide probes based on 2,20-dipyridylaminoanthracene-Cu2+ ensembles, Chem. Commun. 48 (2012) 11513-11515. |
| [5] | F.J. Huo, C.X. Yin, Y.T. Yang, et al., Ultraviolet-visible light (UV-vis)-reversible but fluorescence irreversible chemosensor for copper in water and its application in living cells, Anal. Chem. 84 (2012) 2219-2223. |
| [6] | L.J. Qu, C.X. Yin, F.J. Huo, Y.B. Zhang, Y.Q. Li, A commercially available fluorescence chemosensor for copper ion and its application in bioimaging, Sens. Actuators B 183 (2013) 636-640. |
| [7] | Y.T. Yang, F.J. Huo, C.X. Yin, et al., Combined spectral experiment and theoretical calculation to study the chemosensors of copper and their applications in anion bioimaging, Sens. Actuators B 177 (2013) 1189-1197. |
| [8] | F.J. Huo, L. Wang, C.X. Yin, et al., The synthesis, characterization of three isomers of rhodamine derivative and their application in copper (II) ion recognition, Sens. Actuators B 188 (2013) 735-740. |
| [9] | L.J. Qu, C.X. Yin, F.J. Huo, et al., A pyridoxal-based dual chemosensor for visual detection of copper ion and ratiometric fluorescent detection of zinc ion, Sens. Actuators B 191 (2014) 158-164. |
| [10] | F.Y. Wu, S.G. Cao, C.X. Xie, A highly selective chemosensor for copper ion based on ICT fluorescence, Chin. Chem. Lett. 23 (2012) 607-610. |
| [11] | X.B. Li, Z.G. Niu, L.L. Chang, M.X. Chen, E.J. Wang, Quinoline-based colorimetric chemosensor for Cu2+: Cu2+-induced deprotonation leading to color change, Chin. Chem. Lett. 25 (2014) 80-82. |
| [12] | K. Yoosaf, B.I. Ipe, C.H. Suresh, K.G. Thomas, In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media, J. Phys. Chem. C 111 (2007) 12839-12847. |
| [13] | T. Gunnlaugsson, J.P. Leonard, N.S. Murray, Highly selective colorimetric nakedeye Cu(II) detection using an azobenzene chemosensor, Org. Lett. 6 (2004) 1557-1560. |
| [14] | M.H. Lee, B.K. Cho, J. Yoon, et al., Selectively chemodosimetric detection of Hg(II) in aqueous media, Org. Lett. 9 (2007) 4515-4518. |
| [15] | M. Zhu, M.G. Yuan, X.F. Liu, et al., Visible near-infrared chemosensor for mercury ion, Org. Lett. 10 (2008) 1481-1484. |
| [16] | S.J. Lee, S.S. Lee, I.Y. Jeong, et al., Azobenzene coupled chromogenic receptors for the selective detection of copper(II) and its application as a chemosensor kit, Tetrahedron Lett. 48 (2007) 393-396. |
| [17] | R.L. Sheng, P.F. Wang, W.M. Liu, et al., A new colorimetric chemosensor for Hg2+ based on coumarin azine derivative, Sens. Actuators B 128 (2008) 507-511. |
| [18] | H.L. Mu, R. Gong, Q. Ma, Y.M. Sun, E.Q. Fu, A novel colorimetric and fluorescent chemosensor: synthesis and selective detection for Cu2+ and Hg2+, Tetrahedron Lett. 48 (2007) 5525-5529. |
| [19] | T. Elisa, M.N. Elizabeth, J. Jacek, S.J. Lippard, Organelle-specific zinc detection using zinpyr-labeled fusion proteins in live cells, J. Am. Chem. Soc. 130 (2008) 15776-15777. |
| [20] | B. Tang, H. Huang, K.H. Xu, et al., Highly sensitive and selective near-infrared fluorescent probe for zinc and its application to macrophage cells, Chem. Commun. 34 (2006) 3609-3611. |
| [21] | M.N. Elizabeth, J.L. Stephen, Turn-on and ratiometric mercury sensing in water with a red-emitting probe, J. Am. Chem. Soc. 129 (2007) 5910-5918. |
| [22] | P. Li, X. Duan, Z.Z. Chen, et al., A near-infrared fluorescent probe for detecting copper(II) with high selectivity and sensitivity and its biological imaging applications, Chem. Commun. 47 (2011) 7755-7757. |
| [23] | T.R. Li, Z.Y. Yang, Y. Li, et al., A novel fluorescein derivative as a colorimetric chemosensor for detecting copper(II) ion, Dyes Pigments 88 (2011) 103-108. |
| [24] | R. Martínez, A. Espinosa, A. Tárraga, P. Molina, Bis(indolyl)methane derivatives as highly selective colourimetric and ratiometric fluorescent molecular chemosensors for Cu2+ cations, Tetrahedron 64 (2008) 2184-2191. |
| [25] | N. Aksuner, E. Henden, I. Yilmaz, A. Cukurovali, Selective optical sensing of copper(II) ions based on a novel cyclobutane-substituted Schiff base ligand embedded in polymer films, Sens. Actuators B 134 (2008) 510-515. |
| [26] | J.W. Karr, V.A. Szalai, Role of aspartate-1 in Cu(II) binding to the amyloid-b peptide of alzheimer's disease, J. Am. Chem. Soc. 129 (2007) 3796-3797. |
| [27] | J. Li, V.N. Uversky, A.L. Fink, Effect of familial parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of humana-synuclein, Biochemistry 40 (2001) 11604-11613. |
| [28] | B.B. Tewari, Studies on complexation in solution with a paper electrophoretic technique [the system copper(II)/cobalt(II) emethioninee penicillamine], J. Chem. Eng. Data 55 (2010) 1779-1783. |
| [29] | L.M. Zhang, J. Lichtmannegger, K.H. Summer, et al., Tracing copper-thiomolybdate complexes in a prospective treatment for Wilson's disease, Biochemistry 48 (2009) 891-897. |
| [30] | C. Kar, M.D. Adhikari, B.K. Datta, et al., A CHEF-based biocompatible turn ON ratiometric sensor for sensitive and selective probing of Cu2+, Sens. Actuators B 188 (2013) 1132-1140. |
| [31] | X.F. Yang, D.B. Wu, H. Li, Sensitive determination of cobalt(II) using a spiro fluorescein hydrazide as a chemiluminogenic reagent, Microchim. Acta 149 (2005) 123-129. |
| [32] | H.A. Benesi, J.H. Hildebrand, A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons, J. Am. Chem. Soc. 71 (1949) 2703-2707. |
| [33] | M. Barra, C. Bohne, J.C. Scaiano, Effect of cyclodextrin complexation on the photochemistry of xanthone. Absolute measurement of the kinetics for tripletstate exit, J. Am. Chem. Soc. 112 (1990) 8075-8079. |
| [34] | S.Q. Cui, S.Z. Pu, W.J. Liu, G. Liu, Synthesis and photochromic properties of a multiple responsive diarylethene and its selective binding affinity for copper(II) ion, Dyes Pigments 91 (2011) 435-441. |
| [35] | Y.B. Ding, X. Li, T. Li, W.H. Zhu, Y.S. Xie, α-Monoacylated and α,α'-and α,β'-diacylated dipyrrins as highly sensitive fluorescence "Turn-on" Zn2+ probes, J. Org. Chem. 78 (2013) 5328-5338. |
| [36] | B. Chen, Y.B. Ding, X. Li, et al., Steric hindrance-enforced distortion as a general strategy for the design of fluorescence "turn-on" cyanide probes, Chem. Commun. 49 (2013) 10136-10138. |
| [37] | B.P. Joshi, J. Park, W.I. Lee, et al., Ratiometric and turn-on monitoring for heavy and transition metal ions in aqueous solution with a fluorescent peptide sensor, Talanta 78 (2009) 903-909. |
| [38] | M.J. Kim, K. Kaur, N. Singh, D.O. Jang, Benzim idazole-based receptor for Zn2+ recognition in a biological system: a chemosensor operated by retarding the excited state proton transfer, Tetrahedron 68 (2012) 5429-5433. |
| [39] | S.H. Mashraqui, R. Betkar, S. Ghorpade, et al., A new internal charge transfer probe for the highly selective detection of Zn(II) by means of dual colorimetric and fluorescent turn-on responses, Sens. Actuators B 174 (2012) 299-305. |
| [40] | C.J. Gao, X.J. Jin, X.H. Yan, et al., A small molecular fluorescent sensor for highly selectivity of zinc ion, Sens. Actuators B 176 (2013) 775-781. |




