Recently,multicomponent reactions (MCRs) have been shown to provide an efficient access to highly complex products in a single step. They have proven to be fast,convergent,atom-efficient reactions and often required effortless purifications through give generally high yields of products [1]. These reactions have emerged as a valuable tool in the synthesis of drug libraries because they have greater advantages over conventional strategies to synthesize biologically active compounds with significant structural diversity [2]. Consequently, designing novel MCRs to generate of diverse ‘‘drug-like’’ molecules has been the focus of many medicinal chemists.
Since the discovery of norfloxacin by Kogaet al. in the early 1980s [3],fluoroquinolones have been used extensively in clinic because of their extremely potent activity,rapid bactericidal effects,and low incidence of resistance development [4]. These antibiotics exhibit properties like excellent bioavailability and a relatively low incidence of adverse and toxic effects [5]. Also a large number of fluoroquinolone derivatives have been designed and synthesized,and structure activity relationship (SAR) has been accumulated. Cyclopropyl (Ciprofloxacin) and mono/difluorophenyl (Difloxacin or Temafloxacin) groups are generally considered the most favorable substituents at the N-1 position of the 4-quinolones [6, 7].
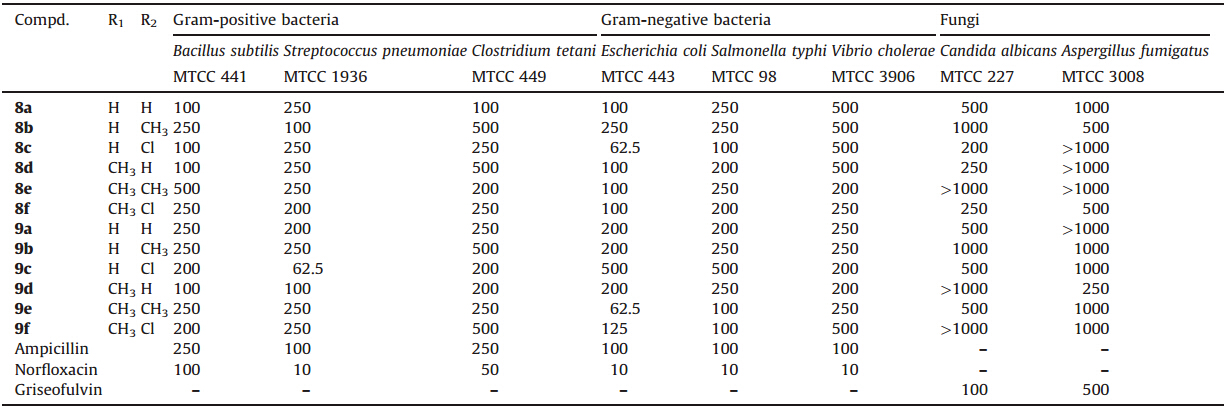
Over the past few years,we have been principally engrossed in the synthesis of quinolone-containing structures for biological evaluations [8, 9, 10, 11] on the fact that the quinoline moiety is found in a large variety of naturally occurring compounds diverse bioactivities such as antibiotic [12],antimalarial [13],antituberculosis [14],anti-HIV [15],anticancer [16],antihypertensive properties [17]. Additionally,some known fluoroquinolones antibiotics such as Ciprofloxacin,Delafloxacin,Sparfloxacin, Temafloxacin,Difloxacin and Norfloxacin are well known drugs. One of the best known drugs with a trifluoromethyl-quinoline nucleus,the antimalarial drug mefloquine is still being used today [18]. Also,a 5-quinolone derivative,2-(benzylthio)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-3-carbonitrile act as mGlur1 Modulator [19] (Fig. 1).

|
Download:
|
| Fig. 1. Example of biologically active quinoline derivatives. | |
In this study,we introduce substituted 2-thiophenoxyquinolines at the C-4 position and lipophilic groups,trifluoromethyl substituted aromatic ring at theN-1 position on 5-quinolone to probe biological activity. The synthesis ofN-aryl quinolone bearing 2-thiophenoxyquinolines derivatives was based on the assumption that the incorporation of more than one bioactive moiety into a single scaffold may produce novel heterocycles with good antimicrobial activity. 2. Experimental
All the reagents were obtained commercially and used without
further purification. Solvents used were of analytical grade. All
melting points were taken in open capillaries and were uncorrected.Thin-layer chromatography (TLC,on aluminum plates coated with
silica gel 60 F254,0.25 mm thickness) (Merck,Darmstadt,Germany)
was used for monitoring the progress of all reactions,purity and
homogeneity of the synthesized compounds. UV radiation and/or
iodine were used as the visualizing agents. Elemental analysis (% C,
H,N) was carried out using a Perkin-Elmer 2400 series-II elemental
analyzer (Perkin-Elmer,USA) and all compounds are within±0.4% of
theoretical values. The IR spectra were recorded in KBr on a PerkinElmer Spectrum GX FT-IR Spectrophotometer (Perkin-Elmer,USA) and
only the characteristic peaks are reported in cm-1.
1H NMR and
13C NMR spectra were recorded in DMSO-d6 on a Bruker Avance 400F
(MHz) spectrometer (Bruker Scientific Corporation Ltd.,Switzerland)
using solvent peak as an internal standard at 400 MHz and 100 MHz
respectively. Chemical shifts are reported in parts per million (ppm).
Mass spectra were scanned on a Shimadzu LCMS 2010 spectrometer
(Shimadzu,Tokyo,Japan).
The synthetic approach adopted to obtain 6-(un)substituted-2-((4-(un)substituted phenyl)thio)quinoline-3-carbaldehydes 4a-f
is shown in Scheme 1. The starting material 2-chloro-3-formyl
quinolines 2a-b were prepared by the Vilsmeier-Haack reaction
[20] from acetanilides1a-band were conveniently converted into
4a-f by nucleophilic displacement of chloro group at C-2 in1a-d with 4-(un)substituted thiophenols 3a-c in the presence of
anhydrous k2CO3in DMF at 120°C for 2 h.
The required &bate;-enaminones 5 were prepared by the reaction of
5,5-dimethylcyclohexane-1,3-dione with trifluorinated aromatic
amines according to the literature procedures [10]. Subsequently,
the one-pot three-component cyclocondensation of a series of 4a-
f, &bate;-enaminones 5 and malononitrile 6 or isopropyl cyanoacetate 7
in ethanol containing a catalytic amount of piperidine afforded the
targeted compounds 8a-f and 9a-f in good to excellent yields.
A plausible mechanism for the reaction is provided in Scheme 2.
The heterylidenenitrile,containing an electron-poor C55C double
bond is produced,from the Knoevenagel condensation between
4a-f and malononitrile 6 or isopropyl cyanoacetate 7 followed by
dehydration. Michael addition of &bate;-enaminone 5 to the ylidenic
bond forms an acyclic intermediate,which cyclizes by nucleophilic
attack of the NH group on the cyano carbon. The subsequent
tautomerisation gives the final products 8a-f and 9a-f .
The MICs of synthesized compounds 2'-amino-7',7'-dimethyl-5'-oxo-2-((4-sub)phenylthio)-1'-(3-(trifluoromethyl)phenyl)-1',4',5',6',7',8'-hexahydro-[3,4'-biquinoline]-3'-carbonitrile 8a-f and
isopropyl 2'-amino-7',7'-dimethyl-5'-oxo-2-((4-sub)phenylthio)-1'-(3-(trifluoromethyl)phenyl)-1',4',5',6',7',8'-hexahydro-[3,4'-biquinoline]-3'-carboxylate 9a-f against three Gram-positive and three
Gram-negative bacteria as well as two fungi were carried out by the
broth microdilution method according to National Committee for
Clinical Laboratory Standards (NCCLS) [21].
3. Results and discussion
The values of the MIC against microorganisms showed that
these compounds have significant inhibitory effects. The antibacterial data indicate that the synthesized compounds are more
effective against Gram-positive strains. It shows that lipophilic
character of the molecules plays an important role in their
antimicrobial effect. Among them compound 9c was found to be
more active than the comparator against Gram-positive bacterium
S. pneumoniae. Compounds 8a,8c,8d and 8d showed promising
activity againstB. subtilis. Compounds 8b and 9d showed moderate
activity against S. pneumoniae. Compounds 8a exhibited good
activity towardC. tetani. Compound 8c,9e and 9f were found to be
equipotent against Gram-negative bacterium S. typhi. Except
compounds 8b and 9b(R1= H and R2 =CH3),all the compounds
displayed good to excellent inhibitory effects againstC. tetani. Also,
compounds 8c and 9e exhibited significant potency against Gram
negative bacterium E. coli as benchmarked by ampicillin
(MIC = 100ug/mL).
In vitro antifungal activity of the synthesized quinolyl-quinolone derivatives are summarized in Table 1. Compound 9d was endowed promising activity,while the compounds 8b and 9f
showed moderate activity against A. fumigatus. Unfortunately,
none of the synthesized compounds were found sufficiently potent
in inhibiting fungal pathogen C. albicans.
In conclusion,the aim of the present investigation was to design
and synthesis of 5-quinolone derivatives by introducing substituted 2-thiophenoxyquinolines at the C-4 position and diversely
trifluoromethyl substituted phenyl ring at N-1 position to probe
antimicrobial activity. Modification of substituents on both 2-thiophenoxyquinolines ring and N-aryl quinolone ring with
various electron donatig and electron withdrawing groups
improved the activity. Compounds 8c,9c and 9e exhibited
excellent antimicrobial activity. Finally,these compounds represent new scaffolds that could be further optimized to produce more potent and selective antimicrobial agents.
Acknowledgments
The authors are thankful to Prof. H. S. Patel,Head,Department
of Chemistry,Sardar Patel University for providing
1H NMR,13C NMR spectroscopy and research facilities. We are also thankful to
PURSE central facility for mass spectrometry sponsored by DST,
New Delhi,Vaibhav Laboratories,Ahmedabad,Gujarat,India for
the FT-IR,SICART,Vallabh Vidyanagar,for elemental analysis and
Dhanji P. Rajani,Microcare Laboratory,Surat,Gujarat,India for
antimicrobial and antituberculosis screening of the compounds
reported herein. One of the authors (M.B. Kanani) is grateful to
UGC,New Delhi for Research Fellowship in Sciences for Meritorious Students.
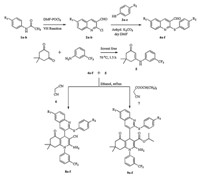
Scheme 1. General synthetic route for the title compounds 8a-f and 9a-f.
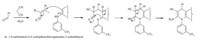
Scheme 2. Plausible mechanistic pathway for the synthesis of 8a-f and 9a-f biquinoline.
![]()
Table 1
Antimicrobial activity results of compounds 8a-f and 9a-f against various microorganisms (MIC,mg/mL).
| [1] | (a) J. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005; (b) H. Bienayme, C. Hulme, G. Oddon, P. Schmitt, Maximizing synthetic efficiency: multi-component transformations lead the way, Chem. Eur. J. 6 (2000) 3321-3329; (c) A. Dömling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39 (2000) 3168-3210. |
| [2] | Z. Huang, Y. Hu, Y. Zhou, D. Shi, Efficient one-pot three-component synthesis of fused pyridine derivatives in ionic liquid, ACS Comb. Sci. 13 (2011) 45-49. |
| [3] | H. Koga, A. Itoh, S. Murayama, S. Suzue, T. Irikura, The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vivo, J. Med. Chem. 23 (198') 1358-1365. |
| [4] | D.C. Hooper, J.C. Wifson, The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans, Antimicrob. Agents Chemother. 28 (1985) 716-721. |
| [5] | P.C. Sharma, A. Jain, S. Jain, R. Pahwa, M.S.J. Yar, Ciprofloxacin: review on developments in synthetic, analytical, and medicinal aspects, Enzyme Inhib. Med. Chem. 25 (2009) 577-583. |
| [6] | M.L. Liu, H.Y. Guo, Evolution of the quinolones antibacterial agents, Yakushigaku Zasshi 27 (2006) 69-76. |
| [7] | (a) J.A. Makawana, M.P. Patel, R.G. Patel, Synthesis and in vitro antimicrobial activity of N-arylquinoline derivatives bearing 2-morpholinoquinoline moiety, Chin. Chem. Lett. 23 (2012) 427-430; (b) H.G. Kathrotiya, M.P. Patel, Synthesis and identification of β-aryloxyquinoline based diversely fluorine substituted N-aryl quinolone derivatives as a new class of antimicrobial, antituberculosis and antioxidant agents, Eur. J. Med. Chem. 63 (2013) 675-684. |
| [8] | (a) H.G. Kathrotiya, N.A. Patel, R.G. Patel, M.P. Patel, An efficient synthesis of quinolinyl substituted imidazole-5-one derivatives catalyzed by zeolite and their antimicrobial activity, Chin. Chem. Lett. 23 (2012) 273-276; (b) D.C. Mungra, M.P. Patel, D.P. Rajani, R.G. Patel, Synthesis and ide ntification of β-aryloxyquinolines and their pyrano[3,2-c]chromene derivatives as a new class of antimic robial and antituberculosis agents, Eur. J. Med. Chem. 46 (2011) 4192-4200. |
| [9] | N.M. Shah, M.P. Patel, R.G. Patel, New N-arylamino biquinoline derivatives: synthesis, antimicrobial, antituberculosis, and antimalarial evaluation, Eur. J. Med. Chem. 54 (2012) 239-247. |
| [10] | H.H. Jardosh, M.P. Patel, Design and synthesis of biquinolone-isoniazid hybrids as a new class of antitubercular and antimicrobial agents, Eur. J. Med. Chem. 65 (2013) 348-359. |
| [11] | J.A. Makawana, D.C. Mungra, M.P. Patel, R.G. Patel, Microwave assisted synthesis and antimicrobial evaluation of new fused pyran derivatives bearing 2-morpholinoquinoline nucleus, Bioorg. Med. Chem. Lett. 21 (2011) 6166-6169. |
| [12] | (a) W.D. Wilson, M. Zhao, S.E. Patterson, et al., Design of RNA interactive anti-HIV agents: unfused aromatic intercalators, Med. Chem. Res. 2 (1992) 102-110; (b) J.X. Wang, Q. Guo, Y. Chai, et al., Synthesis and in vitro antibacterial activities of 7-(4-alkoxyimino-3-hydroxypiperdin-1-yl)quinolone derivatives, Chin. Chem. Lett. 21 (2010) 55-58. |
| [13] | P.A. Leatham, H.A. Bird, V. Wright, D. Seymour, A. Gordon, A double blind study of antrafenine, naproxen and placebo in osteoarthrosis, Eur. J. Rheumatol. Inflamm. 6 (1983) 209-211. |
| [14] | L. Strekowski, J.L. Mokrosz, V.A. Honkan, et al., Synthesis and quantitative structure-activity relationship analysis of 2-(aryl or heteroaryl)quinolin-4-amines, a new class of anti-HIV-1 agents, J. Med. Chem. 34 (1991) 1739-1746. |
| [15] | W.A. Denny, W.R. Wilson, D.C. Ware, et al., U.S. Patent 7,064,117 (2006). |
| [16] | (a) K. Muller, C. Faeh, F. Diederich, Fluorine in pharmaceuticals: looking beyond intuition, Science 317 (2007) 1881-1886; (b) S. Purser, P.R. Moore, S. Swallow, V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev. 37 (2008) 320-330. |
| [17] | T. Nagai, G. Nishioka, M. Koyama, et al., Reactions of trifluoromethyl ketones. IX. Investigation of the steric effect of a trifluoromethyl group based on the stereochemistry on the dehydration of trifluoromethyl homoallyl alcohols, J. Fluorine Chem. 57 (1992) 229-234. |
| [18] | K.J. Palmer, S.M. Holliday, R.N. Brogden, Mefloquine: a review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy, Drugs 45 (1993) 430-475. |
| [19] | (a) J.P. Rocher, B. Bonnet, C. Bolea, et al., mGluR5 negative allosteric modulators overview: a medicinal chemistry approach towards a series of novel therapeutic agents, Curr. Top. Med. Chem. 11 (2011) 680-695; (b) A.L. Rodriguez, M.D. Grier, C.K. Jones, et al., Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity, Mol. Pharmacol. 78 (2010) 1105-1123. |
| [20] | O. Meth-Cohn, N.A. Bramha, A versatile new synthesis of quinolines, thienopyridine and related fused pyridines, Tetrahedron Lett. 23 (1978) 2045-2048. |
| [21] | National Committee for Clinical Laboratory Standards (NCCLS), 940, West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898, USA. Performance standards for antimicrobial susceptibility testing; Twelfth Informational Supplement (ISBN 1-56238-454-6), M100-S12 M7 (2002). |