1. Introduction
Layered double hydroxides (LDHs) have excellent cell transmission and controlled release properties [1, 2, 3, 4, 5, 6, 7, 8, 9, 10],which can be used in the biomedical field to deliver certain biological molecules and specific drugs.Magnetic layered double hydroxide (MLDH) is a new targeted drug carrier invented with a coprecipitation reaction of paramagnetic ions [11, 12],on which the "dextranmagnetic layered double hydroxide-fluorouracil" (DMF) delivery system was established [13]. The magnetic targeting and slowrelease effect of the DMF system have been validated by a series of in vitro and in vivo experiments [14, 15, 16, 17, 18]. To provide further cell biology evidences for theMLDH delivery system,it is necessary to develop a fluorescent marker probe for tracking its celltransmission.
Since the side groups of fluorescent iso-thiocyanate (FITC) can be connected to biomoleculeswithsulfydrylandamido groups through nucleophilic reaction,FITC is widely applied to mark proteins and antibodies as fluorescent dyes [19]. As the study of LDH applied in the biomedical domain goes deeper,researchers have done a lot of work in marking LDH of magnesium,zinc,and aluminum matrices with FITC [20, 21, 22, 23]. The probe mark function of FITC-LDH is based on intercalation assembly,effective transport,and controlled release of FITC by the electropositive brucite laminate of LDH,but the free FITC molecules uncombined with LDH layers can polymerize to isothiocyanate during nucleophilic substitution,which would lead to a false-positive result. Furthermore,FITC,as a popular immunofluorescent marker,is not the best for tracking cell transfer of the LDH system,for its fluorescence is unstable and easy to quench. Fluorescein (C20H12O5,FLU) is very electronegative,highly stable, and strongly fluoresces in reflected or perspective light,which makes it suitable for intercalation assemblywith LDH. Anthony et al. have investigated the properties of MgAl-LDH intercalated with fluorescein [24]. In this paper,we used the magnetic layered double hydroxides [FeII 2FeIII(OH)6][Cl·H2O] [12] as precursor to intercalate fluorescein,synthesizing a magnetic MLDH-FLU probe with ion exchange reaction,and inspected its appropriate pretreatment effects.
2. Experimental 2.1. Synthesis of MLDH precursor
MLDH with chemical formula [FeII 2FeIII(OH)6][Cl·H2O] was prepared by coprecipitation of FeCl2·4H2O and FeCl3·6H2O according to the literature [12]. In detail,61.63 g (0.310 mol) FeCl2·4H2O and 41.91 g (0.155 mol) FeCl3·6H2O were dissolved in 300 mL of water to prepare the mixed salt solution,then 500 mL of NaOH solutions in the concentration 2 mol/L was added under N2 protection and 35 ℃ constant temperature conditions,and the pH of the reaction terminal point was kept at 6.41. The resulting slurry was aged in situ for 45 min,centrifuged with 5000 r/min for 10 min at-10 ℃ to separate the liquid-solid phases,then deposited at low temperature for later use.
2.2. Synthesis of MLDH-FLU fluorescent probe
Fluorescein (6.25 g,0.0188 mol) was wetted with little water, dissolved by concentrated NaOH,diluted with 400 mL water,and adjusted to pH 7.45. The weakly alkaline solution of FLU was added into a 500 mL reactor assembled with a pH electrode and N2 pipeline. After the air inside the reactor was empty and the bath temperature was increased to 35 ℃,6.07 g (0.0188 mol) of MLDH solid was added into the reactor under stirring and circulation of nitrogen,and was kept reacting with FLU for 90 min [25]. 10 mL terminal liquid sample was taken for analysis,and the rest of the slurry was centrifuged in 5000 r/min for 15 min at -10 ℃ to obtain the MLDH-FLU solid product.
2.3. Elution of adsorption pigment on the surface of MLDH-FLU
Small magnon (2× 5 mm) and 8 mL of distilled water were added into the sample tube of MLDH-FLU,and the solid was washed for 10 min at room temperature with a magnetic stirrer in N2 atmosphere and centrifuged with 7000 r/min for 10 min at -10 ℃. To prepare the analysis sample of eluent,the supernatant liquid was filtered through a syringe-driven filter,and 1.0 mL of the filtered solution was diluted to 50.0 mL,and its fluorescence intensity was finally measured at the lmax of 526 nm. The operation of washing and analysis above was repeated 12 times.
2.4. Characterization of MLDH-FLU samples
Powder XRD patterns were obtained by a Rigaku D/Max-rB X-ray powder diffractometer (Cu Ka,l = 0.1542 nm) with scan rate of 28/min andscanrangein5-808. Fourier transforminfraredspectra of the samples were recorded over the range of 400-4000 cm-1 on a TENEOR27 infrared spectrophotometer (Brooke Company of Germany) with 4 cm-1 resolution,using the KBr disk method. The thermal behavior of the samples was examined using thermogravimetric and differential thermogravimetric analyses on a SETSYS- 1750CS thermal analyzer (SETARAMof French) in N2 atmosphere at the temperature range from 16 ℃ to 650 ℃ with a heating rate of 8 ℃/min. TEM images were obtained with a Hitachi H-7560B transmission electron microscope at an accelerating voltage of 80 kV,amplifying 70,000-120,000 times. Elemental analysis was completed on a JMS-5600 LV low vacuum scanning electron microscopy (Japanese electron optical company) assembled JEOL X-ray energy dispersive spectrometer (Kevex companies in USA), with the resolution of 131.7 eV and scan range in 0-5000 eV.
The magnetic properties of MLDH and MLDH-FLU solid samples were evaluated with Ancient Egypt magnetic balance (CTP-II magnetic scales,NanJing Sangli Electronics Equipment Workshop), using CuSO4·5H2O as a standard substance to correct magnetic field intensity. The weight-change of an empty sample cell, standard substance cells,and equal altitude samples to be tested in 300-400 mT magnetic field were measured by a Mettler-Toledo analytical balance (AL 104,Mettler Toledo instrument Shanghai Co.,LTD.),and magnetic susceptibility calculations were made using the relative comparison method.
3. Results and discussion 3.1. Liquid phase pH variation during synthesis course of MLDH and MLDH-FLU
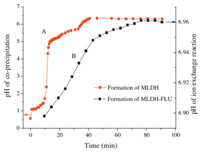
The liquid pH change along with the coprecipitation of MLDH and the ion exchange reaction of MLDH-FLU are shown in Fig. 1. Fig. 1A shows that the formation of MLDH went through two flat pH curves,which increased slowly in the pH range of 1.1-1.7 and 5.0-5.8,respectively. They correspond to the successive precipitation of Fe(OH)3 and Fe(OH)2,similar to the coprecipitation course of MgAl-LDH [26]. Fig. 1 B shows that the liquid phase pH rose along with the ion exchange of MLDH with FLU,implying that the original intercalated ions were replaced gradually. Due to the dissolution of OH- ions displaced by FLU from interlayer of MLDH, OH- concentration of the reaction solution increased slowly,which causes the rise of pH.
|
Download:
|
| Fig. 1.Liquid pH change with reaction course. | |
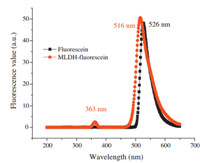
The fluorescent encapsulating rate of MLDH-FLU approached 98.9% that was calculated by the working curve of fluorescein, which indicated that the ion exchange reaction between MLDH and FLU solution was complete. Fig. 2 shows the fluorescence spectra of MLDH-FLU and fluorescein solution. There was a redshift of 10 nm between the maximum emission wavelength of MLDHFLU aqueous solution and that of the intercalating agent fluorescein,and a low emission peak of MLDH-FLU presents at 363 nm of wavelength. Overall,the fluorescence properties of the intercalating agent did not change too much.
|
Download:
|
| Fig. 2.The fluorescence spectra of MLDH-FLU and fluorescein solution. | |
3.2. Solid phase characterization of MLDH and MLDH-FLU
As the MLDH is composed of paramagnetic iron ions,it should present some magnetic features [11, 27, 28]. Table 1 shows the mass magnetisability of MLDH and MLDH-FLU samples tested in the range of 300-400 mT. The magnetic susceptibility of MLDH, original MLDH-FLU,and samples washed less than 7 times are an order of magnitude higher than the standard substance (5.85 × 10-6 cm3/g). These values are enough to easily and quickly manipulate the nanoparticles by external stimulation [14, 16, 18]. The results confirm the most important property for the biomedical application of MLDH,as the superparamagnetic behavior of MLDH is in favor of carrying drugs or fluorochrome to targeted positions under an external magnetic field. The magnetisability of the excessively washed samples is about one over ten times that of the strongly magnetic Fe3O4 but far more than MLDH and original MLDH-FLU,which indicates that partial Fe(OH)2 in MLDH-FLU has become ferroferric oxide by oxidation. This is helpful to improve the particle magnetic responsiveness, but not conducive to maintain its layer structure and controlled release performance. Table 2 shows the element analysis results of MLDH-FLU sample washed 6 times. According to these data the formula of MLDH-FLU can be expressed concretely as [FeII 2FeIII(OH)6·Cl·H2O]·0.8(C20H12O5).
| Table 1 The mass magnetisability of MLDH and MLDH-FLU samples. |
| Table 2 The element analysis results of MLDH-FLU sample. |
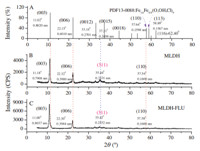
Fig. 3 shows the XRD patterns of MLDH and MLDH-FLU. Fig. 3A represents the XRD of Fe3.6Fe0.9(O,OH,Cl)9,which is the standard model recorded by 13-0088 card of JCPDS (Joint Committee on Powder Diffraction Standards),with the specific diffraction parameters of (0 0 3) (11.02°,0.8020 nm),(0 0 6) (22.15°, 0.4010 nm),(0 0 1 2) (33.14°,0.2701 nm),(0 0 1 5) (37.31°, 0.2408 nm),(1 1 0) (57.64°,0.1598 nm) and (113) (58.89°, 0.1567 nm). Retrieval results by Jade 5.0 showed that the XRD diffraction characteristics of MLDH and MLDH-FLU were tallied well with the 13-0088 standard,demonstrating those crystal forms are belong to R-hexagonal system,a family ramification of the layered double hydroxides. Compared with the standard model, the c axial crystal face parameters of d003 decreased for MLDH but increased for MLDH-FLU,indicating that the interlayer spacing of the MLDH sample was smaller than that of Fe3.6Fe0.9(O,OH,Cl)9, with which intercalation of fluorescent giant molecules caused a mild increase in interlayer space of the MLDH matrix. Both MLDH and MLDH-FLU displayed a ferroferric oxide diffraction peak (3 1 1) at 35.4°,signifying there was trace oxidate contained in these samples. The calculated results of XRD in Table 3 shows that the cell parameters a of MLDH-FLU approached that of the precursor (about 0.32 nm),while the cell parameters c and channel height h increased slightly compared with the precursor,suggesting that the small anions of OH- and Cl- had been replaced by fluorescein molecules [24]. The plane diameter Da of MLDH-FLU particles increased twice over its precursor,together with an increase in the number of cell units contained in particles. Results above show that the intercalation of fluorescein did not alter the crystalline phase characteristics of MLDH,but particle morphology features of the carrier were influenced by recombination,as displayed in the increase in both lateral dimension and longitudinal overlying of the product. In addition,with a redouble in crystallinity,the oxidation index v(Fe3O4) of MLDH-FLU reduced to half of the precursor. It is shown that the fluorescent flat molecule was suitable for intercalation assembly with MLDH. Particles of the product grew along the a-axis and packed orderly along the c-axis,and the stability,antioxidant capacity,and crystallinity of the composited system increased due to this perfect geometric matching.
|
Download:
|
| Fig. 3.XRD pattern of MLDH and MLDH-FLU. | |
| Table 3 XRD calculated result of MLDH and MLDH-FLU samples. |
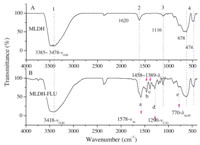
Fig. 4 shows FT-IR of the samples. MLDH displayed the specific absorption of layered double hydroxide,as shown in Fig. 4A marked by 1-4. The absorption peak 1 at 3385-3477 cm-1 is assigned to the stretching vibration νO-H of hydroxide layer and interlayer water molecules,absorption peak 2 at 1620 cm-1 is due to the bending vibration dOH of LDH laminate,while the peak 3 at 1116 cm-1 is due to the deformation vibration δMO-H of the hydroxyl hydrogen atoms on the surface of the LDH layer,and the peak 4 at 678 cm-1 and 474 cm-1 can be attributed to the stretching vibration νM-O of metal-oxygen bonds within LDH layer board [2, 5, 6, 7, 12]. Those specific absorptions of the precursor were kept by its intercalated product,as demonstrated by the peaks at 3418 cm-1,1114 cm-1,635 cm-1,and 471 cm-1 in Fig. 4B,with the same affiliation as νO-H,δO-H and νMO-H of MLDH matrix. The absorption of dOH (corresponding to peak 2 in Fig. 4A) was annexed to nCO-O- absorption of open chaining fluorescent molecule,and was covered by the strong wide band nearby 1578 cm-1. The new absorption peaks of MLDH-FLU recorded at 1578 cm-1,1458 cm-1, 1389 cm-1,1290 cm-1,and 770 cm-1 with marker of a,b,c,d,and e,can be successively assigned to the benzene ring vibration nAr of fluorescein,bending δC-H of methylene hydrogen atoms,the νC-O mode of aromatic ester bond and wagging vibration δAr-H of benzene ring hydrogen atoms. MLDH-FLU inherited the characteristic absorption of the precursor and showed the specific band of intercalated fluorescein,indicating that MLDH-FLU was a new composite assembled with fluorescein (C20H12O5) and MLDH.
|
Download:
|
| Fig. 4.IR spectrum of MLDH (A) and MLDH-FLU (B). | |
3.3. Elution effect of adsorbed pigment on surface of MLDH-FLU
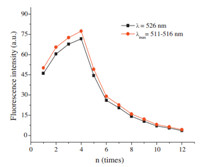
To eliminate possible false positive results caused by the invasion of free fluorescein,the pigments absorbed on the surface of MLDH-FLU probe need to be cleared. Fig. 5 shows the fluorescence variation trend of the eluate during washing cycles.Fluorescent intensity of the eluate reached the maximum when washing times n = 4,and then declined with increase of washing times. The drop rate of the I-n curve slowed down significantly at n = 6-7,indicating that fluorescein adsorbed on the surface of MLDH-FLU had been cleared mainly when n = 7. Judging from the fluorescence intensity of eluate,the ideal elution times should be controlled between 5 and 6. Excessive washing might increase the opportunities for contact oxidation of the samples by air or water soluble O2. On the other hand,the intercalated fluorescein would be released partly from the interlayer of MLDH under washing conditions,which may weaken the marked effect of MLDH-FLU as fluorescent probe.
|
Download:
|
| Fig. 5.Fluorescence intensity variation of eluant. | |
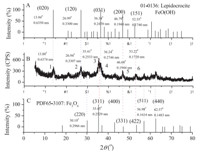
Fig. 6 shows the XRD analysis for possible phase changes caused by excessive washing. Fig. 6A and C give the XRD model of JCPDS standard card 01-0136 and 65-3107 in the spectral library of Jade 5.0. They express respectively lepidocrocite FeO(OH) of Amam (63) crystal system and magnetic iron oxide Fe3O4 of Fd-3m cubic system. XRD of the samples washed less than 7 times were almost identical with the original product,except slight decreases in crystallinity and diffracted intensity of (0 0 3) peak. However,the characteristic peaks of MLDH for the sample washed more than 8 times trailed off gradually,together with an increase of the signalto- noise of the baseline. As shown in Fig. 6B,a complicated multipeak diffraction was found in the sample washed 12 times,with the diffraction parameters of (13.888,0.6374 nm),(26.94°, 0.3307 nm),(35.41°,0.2533 nm),(36.24°,0.2744 nm),(46.68°, 0.1944 nm),and (53.22°,0.1720 nm). The peaks marked with 1,2,4 coincided with the (0 2 0),(1 2 0),and (0 3 1) diffraction of the FeO(OH) crystalline phase,while diffraction peak 3 coincided with the (3 1 1) peak of Fe3O4. The peak 5 was overlapped by (2 0 0) diffraction of FeO(OH) and (3 3 1) of Fe3O4,and the peak 6 coincided with (1 5 1) peak of FeO(OH) and (4 2 2) of Fe3O4. The diffraction feature of Fe3O4 was more obvious than that of FeO(OH) when comparing diffraction peak 3 with 4,and the (2 2 0),(5 1 1), and (4 4 0) diffractions of the Fe3O4 phase clearly appeared at the corresponding positions in XRD of the excessive washed sample. It is clear that excessive washing of MLDH-FLU would result a change to the hexagonal crystal phase of MLDH,forming a mixed crystal phase composed by FeO(OH) and Fe3O4,in which the proportion of oxidate was higher. The probable reasons for phase transformation of MLDH-FLU may be due to the oxidation by the trace O2 introduced from the open environment and distilled water. In detail,a part of Fe2+ in [FeII 2FeIII(OH)6]+ within the layers of MLDH was oxidized into Fe3+ to form the flaky crystal phase of FeO(OH), and the other parts of Fe2+ were segregated and restructured with Fe3+ to form Fe3O4. In other words,the R-hexagonal structure of MLDH was partly transformed into Amam (63) and Fd-3m space groups under excessively washed conditions. The calculated results of XRD show that the plane dimension of particles of the solid sample washed 12 times dropped to 1/4 that of the original product,and their longitudinal accumulation degree dropped by 1/ 2,with a rise of oxidation phase proportion to 80.18%. In conclusion,the ideal elution times for pigments adsorbed on surface of the MLDH-FLU original product should be controlled between 5 and 6 limits.
|
Download:
|
| Fig. 6.XRD pattern of excessive elution solid phase sample of MLDH-FLU. | |
3.4. Morphology characterization and thermogravimetric analysis of solid phase samples
Fig. 7A shows the particle morphology of MLDH-FLU original product in a view field magnified 15,000 times,where some large hexagon particles with side length in 104-113 nm and thickness in 17 nmwere found. Compared with precursor (TEM not shown),the regularity of hexagon morphology,the particle size,and laminated thickness of the MLDH-FLU original product increased observably, which is consistent with the fact that the crystallinity and (0 0 3) diffraction intensity of MLDH-FLU was far higher than that of MLDH. TEM together with XRD show that the fluorescein molecule with a triple six electronic conjugated hexatomic ring structure is a kind of ideal intercalation object with MLDH,as it is able to keep and strengthen the hexagon stacking order of the MLDH layers,and promote the plane and vertical growth of the MLDH-FLU supermolecule during intercalation assembly. Fig. 7B shows TEM image of the sample washed 6 times in a view field magnified 20,000 multiples. The single particle showed a clear oval hexagonal outline,and the average particle size of the washed sample was 22 nm which was less extreme than that of the original product. The scatter uniformity of the washed sample increased significantly compared with the original product,but there was still an aggregated particle of hexagon shape in the center of Fig. 7B with a side length of 69 nm and thickness of 18 nm,displaying the aggregation habits of the MLDH type particle. The aggregation behavior of the MLDH particle showed an obvious trend of hexagon orientation,which is identical with the trait of the LDH cell,a typical lineage of hexagonal crystal systems. Fig. 7C shows the irregular particle morphology of an excessively washed sample (12 times) in the view field magnified 30,000 times. Except for a massive diamond shaped particle of side length 132 nm,many of the particles became fibrous with poor regularity and uniformity. Some of the strip may be the feature morphology of MLDH-FLU particle when perpendicular to the membrane-based surface of the TEM sample shelf,reflecting the longitudinal dimension and lamellar accumulation of the layered particles. For instance,a smaller particle in the top left corner of the figure was found with a stacking thickness of 44 nm. The other part of the strip morphology was probably the transformed crystal phase of MLDH concerned with the fibrous crystal of FeO(OH). Results of TEM and XRD show that excessive washing would cause reduction of configuration regularity of MLDH-FLU particles,decrease crystallinity,and increase oxidation degree. These indicate that the MLDH type compounds should be synthesized and treated under the conditions free from O2.

|
Download:
|
| Fig. 7.TEM image of MLDH-FLU original product (A) and elution solid phase sample (B and C). | |
The thermogravimetric analysis of MLDH,MLDH-FLU (sample washed 6 times) and its excessively washed sample (12 times) are shown in Fig. 8A-C,respectively. For the precursor,the first thermal event occurred in the region of 60.4-123.5 ℃ and is attributed to the removal of physisorbed water on the external surface of MLDH,which corresponds to a sharp endothermic peak in DSC curve at 99.4 ℃ with 46.63% of weight loss and 791.2 J/g of enthalpy. The second weight loss occurred in the interval of 204.0- 260.5 ℃ with 1.77% of weight less and 22.93 J/g of enthalpy at the peak temperature 235.2 ℃,which can be attributed to the anhydration of interlayer structure water [4, 26]. The third thermal event occurred in the interval of 290.4-411.2 ℃ with 0.15% of weightlessness rate and 791.2 J/g of enthalpy at 352.2 ℃ and is due to the complex course including hydroxyl substitution of MLDH laminate by intercalated Cl- and OH- anion,anhydration decomposition of layered hydroxide and depth decomposition of LDO complex [5, 26]. Fig. 8B shows that the decomposition of MLDH-FLU progressed through three major stages of weight loss. These occurred respectively at the maximal temperatures of 105.0 ℃,234.2 ℃ and 335.2 ℃,with weight losses of 64.19%,1.42% and 0.98% respectively. A more complicated thermal behavior than that of precursor MLDH occurred in the region of 280-600 ℃. The first stage of weight loss in the range 49.8-156.3 ℃ is due to the removal of physisorbed water and evaporation of free pigment absorbed on the external surface of MLDH,the second stage of weight loss occurred in the region of 191.0-269.1 ℃,corresponding to the desorption of interlayer structure water,and the third stage of weight loss occurred originally in the region of 284.9- 384.2 ℃ due to the anhydration decomposition of layered hydroxide. The third stage was followed by the subsequent events within 383.2-593.0 ℃,corresponding to a broad exothermic peak in the DSC curve at 475.0 ℃ with 2.76% weight loss,which can be attributed to the depth decomposition of the organic object remained in the interlayer of LDO complex [6, 26]. The thermal behavior of the excessively washed sample was significantly different from MLDH-FLU (sample washed 6 times),as shown in Fig. ℃. Specifically,the weight loss in the interval of 49.6-113.5 ℃ was 21.92%,which was only one-thirds to that of the former,and the enthalpy at 93.9 ℃ was 402.6 J/g,which was dropped 1/2 compared with the former,showed that the surface elution had definite removal-water effect. The weight loss in interval of 178.2- 273.9 ℃ was 4.21% which was double to that of the former with an enthalpy of 145.1 J/g at 241.8 ℃,and the weight loss in interval of 320.1-403.5 ℃ was 2.12% with the enthalpy 56.54 J/g at 363.1 ℃. The weight loss in interval of 403.5-603.8 ℃ was 1.56% that was below to the former. The influence of excessive elution for thermal behavior of MLDH-FLU was mainly displayed on the increase of dehydration of the MLDH layers. This is probably due to the phase transformation of MLDH-FLU,which results the decrease in thermal stability of the MLDH layers frame.

|
Download:
|
| Fig. 8.TG-DSC curves of different samples. | |
4. Conclusion
The conjugated fluorescein can be an idealized chromophore to intercalate into the magnetic layered double hydroxide for fluorescent probe application. This intercalation assembly does not affect the internal structure of the MLDH laminate except increase of the interlayer spacing,increasing crystallinity,stability, and anti-oxidation of the composite. MLDH-FLU retains IR characteristics of MLDH and shows functional absorption of the intercalated fluorescein. Thermal analysis proves that the fluorescent object can be deposited steadily in the interlayer of MLDH. The ideal clearing times of the pigments adsorbed on the surface of MLDH-FLU should be controlled in 5-6; excessive washing in oxidation environment may change the R-hexagonal phase of MLDH matrix to lepidocrocite and magnetic iron oxide.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81260483,20961008).
| [1] | J.H. Choy, S.Y. Kwak, J.S. Park, Y.J. Jeong, J. Portier, Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide, J. Am. Chem. Soc. 121 (1999) 1399-1400. |
| [2] | J.H. Choy, S.Y. Kwak, Y.J. Jeong, J.S. Park, Inorganic layered double hydroxides as nonviral vectors, Angew. Chem. Int. Ed. 39 (2000) 4041-4045. |
| [3] | J.H. Choy, J.S. Park, Y.J. Jeong, S.Y. Kwak, Cellular uptake behavior of [gamma-P-32] labeled ATP-LDH nanohybrids, J. Mater. Chem. 11 (2001) 1671-1674. |
| [4] | G.Q. Wan, D.X. Li, C.F. Li, J. Xu, W.G. Hou, From Zn-Al layered double hydroxide to ZnO nanostructure: gradually etching by sodium hydroxide, Chin. Chem. Lett. 23 (2012) 1415-1418. |
| [5] | M.R. Pérez, I. Pavlovic, C. Barriga, et al., Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al layered double hydroxide intercalated with EDTA, Appl. Clay Sci. 32 (2006) 245-251. |
| [6] | G.J. Gou, F.J. Bao, H.P. Xu, Z.X. Zheng, Effects of drug release medium on layered double hydroxide carrier, Acta Phys. Chim. Sin. 24 (2008) 1675-1680. |
| [7] | M.Z. Wang, Y. Li, J.J. Ji, et al., Novel hybrids of Cu2+ ternary complexes of salicylidene-amino acid Schiff base with phenanthroline (or bipyridine) intercalated in Mg/Al-NO3- layered double hydroxide, Chin. Chem. Lett. 24 (2013) 593-596. |
| [8] | G.J. Gou, H.P. Xu, J.P. Liu, Loading and in vitro release of acetylsalicylic acid via layered double hydroxides, Acta Chim. Sin. 67 (2009) 65-82. |
| [9] | K. Ladewig, M. Niebert, Z.P. Xu, P.G. Peter, Q.L. Gao, Controlled preparation of layered double hydroxide nanoparticles and their application as gene delivery vehicles, Appl. Clay Sci. 48 (2010) 280-289. |
| [10] | K.L. Xu, G.M. Chen, J.Q. Shen, Direct decarbonation of micrometer-scale layered double hydroxides without morphology damage, Chin. Chem. Lett. 23 (2012) 805-808. |
| [11] | J.M.R. Génin, C. Ruby, Anion and cation distributions in Fe(II-III) hydroxysalt green rusts from XRD and Mössbauer analysis (carbonate, chloride, sulphate, ...);the "fougerite" mineral, Solid State Sci. 6 (2004) 705-718. |
| [12] | G.J. Gou, F.J. Bao, J.H. Yang, Preparation of Magnetic LDH with a Nitrogen-Hydrogen Preserve Co-Precipitation Method, CN 101607088B, 2009. |
| [13] | G.J. Gou, F.J. Bao, Y.X. Jiang, Preparation of the Magnetic Targeted and Extended Release Tri-Polymer with a Packed Structure of "Dextran-Magnetic LDH-Fluorouracil", CN 101607091B, 2011. |
| [14] | G.J. Gou, Y.H. Liu, Y. Sun, et al., The supra-molecular assembly and magnetic targeted slow-release effect of "dextran-magnetic layered double hydroxidefluorouracil" drug delivery system, Acta Pharm. Sin. 46 (2011) 1390-1398. |
| [15] | G.J. Gou, Z.Y. Wang, Y.H. Liu, et al., The supra-molecular assembly characterization and urgent toxic level of "dextran-magnetic layered double hydroxide-fluorouracil" drug delivery system, Acta Chim. Sin. 70 (2012) 161-169. |
| [16] | J. Huang, G.J. Gou, B. Xue, et al., Preparation and characterization of "dextranmagnetic layered double hydroxide-fluorouracil" targeted liposomes, Int. J. Pharm. 450 (2013) 323-330. |
| [17] | J. Huang, B. Xue, G.J. Gou, Study on cytotoxicity of "dextran-magnetic layered double hydroxide-fluorouracil" targeted liposomes in vitro, Appl. Mech. Mater. 320 (2013) 526-528. |
| [18] | Y.H. Liu, G.J. Gou, Containment proliferation and induction apoptosis effect of DMF drug delivery system on human colon cancer cells SW 480, Chin. Pharm. J. 48 (2013) 359-367. |
| [19] | D. Samuel, R.J. Patt, R.A. Abuknesha, A sensitive method of detecting proteins on dot and western blots using a monoclonal antibody to FITC, J. Immunol. Methods 107 (1988) 217-224. |
| [20] | J.M. Oh, S.J. Choi, S.T. Kim, Cellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: enhanced efficacy due to clathrin-mediated endocytosis, Bioconjugate Chem. 17 (2006) 1411-1417. |
| [21] | D. Yan, J. Lu, M. Wei, D.G. Evans, X. Duan, Sulforhodamine B intercalated layered double hydroxide thin film with polarized photoluminescence, J. Phys. Chem. B 113 (2009) 1381-1389. |
| [22] | J.M. Oh, S.J. Choi, G.E. Lee, J.E. Kim, J.H. Choy, Nanoparticle uptake: inorganic metal hydroxide nanoparticles for targeted cellular uptake through clathrin-mediated endocytosis, Chem.: Asian J. 4 (2009) 67-73. |
| [23] | Z.P. Xu, M. Niebert, K. Porazik, et al., Subcellular compartment targeting of layered double hydroxide nanoparticle, J. Control. Release 130 (2008) 86-94. |
| [24] | A.W. Musumeci, G.M. Mortimer, M.K. Butler, et al., Fluorescent layered double hydroxide nanoparticles for biological studies, Appl. Clay Sci. 48 (2010) 271-279. |
| [25] | G.J. Gou, Y. Sun, F.Y. Zhang, et al., A fluorescent probe constituted with intercalation assembly of magnetic layered double hydroxide and fluorescein, CN 103289678A, 2013. |
| [26] | G.J. Gou, P.H. Ma, M.X. Chu, Kinetics of synthetic of Cl- type hydrotalcite like with coprecipitation reaction, Acta Phys. Chim. Sin. 20 (2004) 1357-1363. |
| [27] | X.Y. Ren, Y. Xue, J. Liang, L.S. Ding, X. Liao, Selective extraction of flavonoids from Ginkgo biloba leaves using human serum albumin functionalized magnetic nanoparticles, Chin. Chem. Lett. 24 (2013) 1099-1102. |
| [28] | M.C. Zhang, Q. Zhou, Y. Zhou, A.M. Li, C.D. Shuang, Efficient adsorption and desorption of Cu2+ by a novel acid-resistant magnetic weak acid resin, Chin. Chem. Lett. 23 (2012) 1267-1270. |







