Combustion catalysts as ballistic modifiers are important ingredients in solid rocket composition [1, 2, 3]. The function of combustion catalysts is to adjust the burning rate and to reduce the pressure exponent of solid propellants. Metal oxides such as ferric oxide and copper chromite are highly effective ballistic modifiers, but they impose a penalty on the energetic of propellant system due to their inert nature. Energetic combustion catalysts [4, 5, 6] are envisaged to achieve the catalytic effect on burning rate without much adverse effect on the energetic of the solid rocket propellants. Therefore,energetic combustion catalysts are preferable over the inert combustion catalysts in solid propellants.
4-Amino-3,5-dinitro-1H-pyrazole (LLM-116) [7, 8, 9] was synthesized initially in 2001. It was a small molecule compound with high energy (the detonation velocity is 9008 m/s) and low mechanical sensitivity (the friction sensitivity is 0%),but the acidity of LLM-116 limits its application in solid rocket composition. So some researchers found that it can be used as a key intermediate for the production of trinitropyrazole [10, 11] and some energetic salts [12, 13]. The copper salt of 4-amino-3,5-dinitropyrazole ([Cu(adnp)2(H2O)2]) was designed and synthesized as a new high energy catalyst,which will be used as an energetic combustion catalyst to adjust the burning properties of solid propellant. Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) is the key energetic material and the most common oxidizer in the solid rocket propellant. Nitrocellulose (NC) is the most commonly used binder in solid rocket propellant. Considering the limited loading of RDX and NC in the rocket,it is crucial to further improve its decomposition efficiency to maximize the energy production and to decrease its burning temperature for easy operation and control. So,the catalysis of RDX and NC thermal decomposition is very important. The studies of the structure,the thermal behaviors and catalytic activity are a very important starting point for the selection,application and exploitation of [Cu(adnp)2(H2O)2]. To the best of our knowledge,these studies have not been reported previously.
The aim of this work is to use the acidity of LLM-116 to synthesize 4-amino-3,5-dinitropyrazole copper salt (Scheme 1), obtain the single crystal of [Cu(adnp)2(H2O)2] using water as solvent,study the non-isothermal decomposition kinetics of ([Cu(adnp)2(H2O)2]) under static state by means of different heating rate differential scanning calorimetry (DSC) [14, 15, 16, 17, 18],and examine the catalytic activity on RDX and NC. 2. Experimental
2.1. Materials
4-Amino-3,5-dinitropyrazole was synthesized according to the procedures reported in Ref. [9]. 2.2. Synthesis
2.2.1. Synthesis of 4-amino-3,5-dinitropyrazole potassium salt (ADNPK) [13]
4-Amino-3,5-dinitropyrazole (1.78 g,0.01 mol) was suspended in 20 mL of H2O then KOH (0.56 g,0.01 mol) in 10 mL H2O was added. The reaction mixture was stirred at 60°C for 1 h,40 mL of methanol was added,and the resulting mixture was slowly cooled to 0°C. Some brown sediments of ADNPK formed and were filtered, washed with methanol and dried under vacuum to give the product (1.82 g,86.26%).
IR (KBr,cm-1):y1570,1372 (-NO2),3446,3341,950 (-NH2), 1638,1441,829,750 (Pyrazol).
Anal. calcd. for C3H4N5O5K (%): C 15.72,H 1.747,N 30.57,K 17.03; found: C 15.69,H 1.740,N 30.09,K 17.10. 2.2.2. Synthesis of 4-amino-3,5-dinitropyrazole copper salt ([Cu(adnp)2(H2O)2])
ADNPK (2.13 g,0.01 mol) was suspended in 20 mL of water, then CuSO4 (1.25 g,0.005 mol) in 10 mL H2O was added. The reaction mixture was stirred at 80°C for 2 h. The resulting solids were filtered and dried in air to give the product obtained (1.91 g, 93.6%).
IR(KBr,cm-1): y1573,1378 (-NO2),3446,3340,952 (-NH2), 1638,1441,829,751 (Pyrazol).
Anal. calcd. For C6H4N10O°Cu (%): C 16.22,H 2.252,N 31.53,Cu 14.41; found: C 16.21,H 2.254,N 31.49,Cu 14.38. 2.3. Determination of the single crystal structure
Single crystals suitable for X-ray measurement were obtained
by slow evaporation of an aqueous solution of the titled compound
at room temperature. Diffraction data were collected on a Bruker
SMART diffractometer with graphite monochromated Mo Ka
radiation ( = 0.071073 nm) in φ and ω scan modes at 296 K.
Absorption corrections were applied using the SADABS program.
The structures were solved by the direct methods and successive
Fourier difference syntheses (SHELXTL-97),anisotropic thermal
parameters for all non-hydrogen atoms were refined by the fullmatrix-block least-squares method on F2
(SHELXTL-97) [19].
Hydrogen atoms were added according to the theoretical models.
Crystal data,experimental details and refinement results were
summarized in Table S1 (Supporting information).
2.4. Thermal experimental instruments and conditions
= 0.071073 nm) in φ and ω scan modes at 296 K.
Absorption corrections were applied using the SADABS program.
The structures were solved by the direct methods and successive
Fourier difference syntheses (SHELXTL-97),anisotropic thermal
parameters for all non-hydrogen atoms were refined by the fullmatrix-block least-squares method on F2
(SHELXTL-97) [19].
Hydrogen atoms were added according to the theoretical models.
Crystal data,experimental details and refinement results were
summarized in Table S1 (Supporting information).
2.4. Thermal experimental instruments and conditions
DSC measurements were carried out on a Model TA-910 USA instruments. The operation conditions were as follows: heating rates,5 K min-1,10 K min-1,15 K min-1and 20 K min-1; sample mass,0.5-1 mg; aluminum sample cell; atmosphere,static nitrogen at 0.1 MPa. 2.5. Catalytic activity test
The obtained Cu(adnp)2(H2O)2 was mixed with RDX in 1:5 (wt.%) and NC in 1:5 (wt.%) respectively and the two mixed samples were used for the DSC experiment. The experiment was performed at a programmed heating rate of 10 K min-1(sample mass,0.5-1 mg; aluminum sample cell; atmosphere,static nitrogen at 0.1 MPa). 3. Results and discussion
3.1. Crystal structure
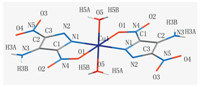
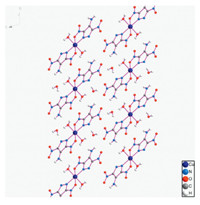
Molecular structure of [Cu(adnp)2(H2O)2] was illustrated in Figs. 1 and 2. Selected bond lengths and bond angles of the titled compound are summarized in Tables S2 (Supporting information). The analytical results indicate that [Cu(adnp)2(H2O)2] crystallizes in triclinic with p-1space group. The molecule of [Cu(adnp)2(H2O)2] contains a copper cation,an organic ligand LLM-116 anion,and two crystal water (Fig. 1). Each copper ion is bonded with four ligands,and the coordination number of copper ion is 6. Two ligands are bidentate ligands and the other two ligands are two crystal water. The bidentate ligand is connected to the copper ion by different coordinating atoms,one of which is the oxygen atom in the nitro group and the other is the nitrogen atom in pyrazole ring.
|
Download:
|
| Fig. 1. The coordination environments of Cu atoms in [Cu(adnp)2(H2O)2]. | |
|
Download:
|
| Fig. 2. The packing molecules of [Cu(adnp)2(H2O)2]. | |
3.2.1. The DSC curve of [Cu(adnp)2(H2O)2]
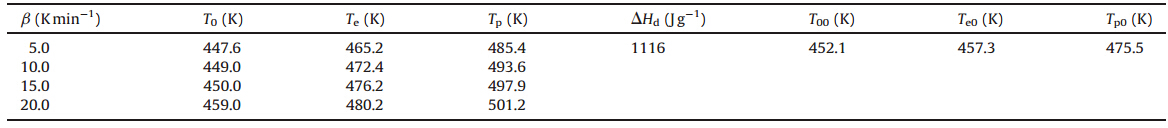
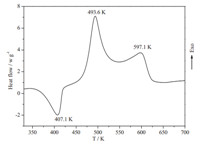
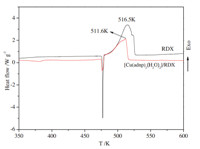
The typical DSC curve (Fig. 3) indicates that the thermal behaviors of [Cu(adnp)2(H2O)2] can be described in three stages. The first stage is the loss of crystal water. The second stage is an obvious exothermic decomposition process. The extrapolated onset temperature,peak temperature and decomposition enthalpy of the process are 472 K,493 K and 1116 J g-1at the heating rate of 10.0 K min-1,respectively. The third stage is the second decomposition of the compound.
|
Download:
|
| Fig. 3. DSC curve for the title compound at a heating rate of 10 K min-1. | |
In order to obtain the kinetic parameters (the apparent activation energy (E) and pre-exponential constant (A)) of the two main exothermic decomposition reactions for the titled compound,a multiple heating method (Kissinger method [20] and Ozawa method [21]) was employed. The Kissinger (1) and Ozawa (2) equations are as follows:

where Tp is the peak temperature (K),bis the linear heating rate (K min-1),Eis the apparent activation energy (kJ mol-1),Ais the pre-exponential constant (s-1),Ris the gas constant (J mol-1 k-1) andCis a constant.
The measured values of the extrapolated onset temperature (Te),peak temperature (Tp) and enthalpy (ΔHd) of the exothermic decomposition reaction were listed in Table 1. The values of Te0 and Tp0in the stage corresponding to b→0 obtained by Eq. (3) were also listed in Table 1 [21].
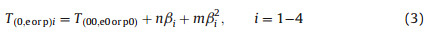
| Table 1 The values ofT0, Te, Tp, DHd, T00, Te0andTp0of the first exothermic decomposition stages for [Cu(adnp)2(H2O)2] determined from the DSC curves at various heating rates (b). |
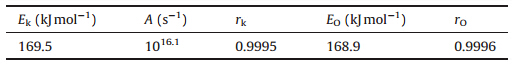
The kinetic parameter values (Eand A) determined by the Kissinger method and the Ozawa method and linear correlation coefficients (r) were listed in Table 2. We can see that the apparent activation energy (E) obtained by the Kissinger method agrees well with that obtained by the Ozawa method,and the linear correlation coefficients are all very close to 1,indicating the satisfactory validity of the results.
| Table 2 The kinetic parameters obtained by the data in Table 1.a |
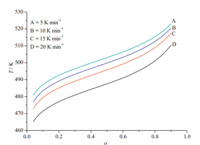
T versus α(the conversion degree) curves at different heating rates [thermal decomposition data of the titled compound by DSC curves] were shown in Fig. 4. By substituting the corresponding data (βi ,Ti and αi,i=1,2,3,...) into Eq. (2),the values ofEfor any given value ofawere obtained and shown in Fig. 5. We can see that the values of E steadily distribute from 156.5 kJ mol-1to 169.7 kJ mol-1in thearange of 0.06-0.60,and the average value ofEis 165.4 kJ mol-1,which is in agreement with that obtained by the Kissinger’s method and Ozawa’s method from only peaktemperature values. So,the values were used to cross examine the validity ofEby other methods.
|
Download:
|
| Fig. 4. T vs. acurves for the decomposition reaction of [Cu(adnp)2(H2O)2]at different heat rates. | |
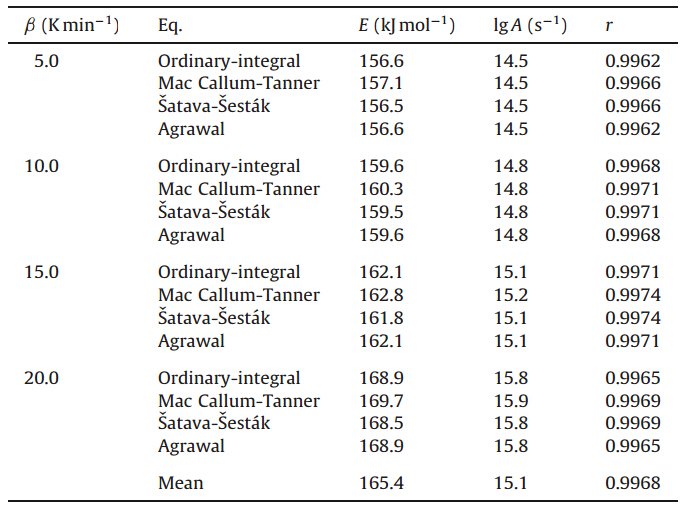
The four integral equations (Ordinary-integral,MacCallumTanner,Šatava-Šesták,Agrawal) were cited to obtain the values of E,A and the most probable kinetic model function (f(a)) from each single DSC curve [22].
Forty-one types of kinetic model functions in Ref. [22] and the corresponding experimental data form DSC curve at different heating rates were fit into the four equations (Ordinary-integral, MacCallum-Tanner,Šatava-Šesták) for calculating. The kinetic parameters (EandA),probable kinetic model function and linear correlation coefficient (r) are presented in Table 3. We can see that the values ofEand lgAobtained by the five equations agree well with each other,and the mean value is close to that obtained by the Kissinger method and the Ozawa method. So,we can conclude that the most probable kinetic model function of the exothermic decomposition reaction can be classified as
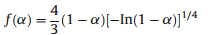
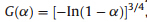 ,according to the unanimity rule of the calculation results from each model equation [22]. So,the kinetic equation of the exothermic decomposition reaction can be
described as:
,according to the unanimity rule of the calculation results from each model equation [22]. So,the kinetic equation of the exothermic decomposition reaction can be
described as: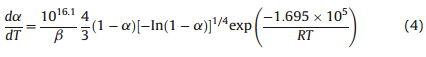
| Table 3 Calculated values of kinetic parameters of decomposition reaction. |
The entropy of activation (ΔS≠),enthalpy of activation (ΔH≠) and free energy of activation (ΔG≠) of the first thermal decomposition process corresponding to T=Tp0,A=Ak and E=Ek obtained by Eqs. (5)-(7) are 59.42 J mol-1 k-1,169.5 kJ mol-1and 1141.26 kJ mol-1,respectively.
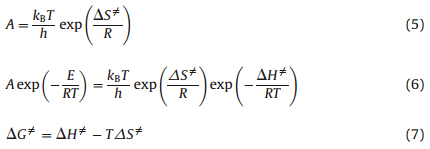


|
Download:
|
| Fig. 6. DSC curves for RDX and [Cu(adnp)2(H2O)2]/RDX at a heating rate of10 K min-1. | |
|
Download:
|
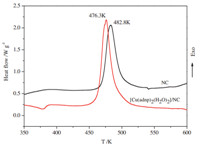
| Fig. 7. DSC curves for NC and [Cu(adnp)2(H2O)2]/NC at a heating rate of 10 K min-1 | |
(1) 4-Amino-3,5-dinitropyrazole copper salt ([Cu(adnp)2(H2O)2]) was synthesized and structurally characterized.
(2) The single crystal of [Cu(adnp)2(H2O)2] was determined by
single crystal X-ray diffraction. The crystal belongs to triclinic
system,space groupp-1with crystal parametersa= 5.541(3) 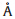 ,β= 7.926(4)
,β= 7.926(4) 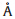 ,c= 10.231(5)
,c= 10.231(5) 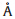 ,β= 101.372(8)°,V= 398.3(3)
,β= 101.372(8)°,V= 398.3(3) 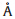 3,Z=1,m= 1.467 mm-1,F(0 0 0) = 243,and Dc = 2.000 g cm-3.
3,Z=1,m= 1.467 mm-1,F(0 0 0) = 243,and Dc = 2.000 g cm-3.
(3) The thermal behavior of the titled compound was studied by DSC. The enthalpy,apparent activation energy and preexponential constants of the exothermic decomposition reaction are 1116 J g-1,169.5 kJ mol-1and 10 16.1 s-1,respectively. The kinetic equation of the exothermic decomposition reaction:
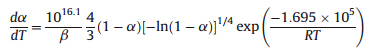
(4) The entropy of activation (ΔS≠),enthalpy of activation (ΔH≠), free energy of activation (ΔG≠),the self-accelerating decomposition temperature (TSADT ) and the critical temperature of thermal explosion (Tb) for [Cu(adnp)2(H2O)2] are 59.42 J mol-1 k-1,169.5 kJ mol-1,1141.26 kJ mol-1,457.3 K and 468.1 K,respectively. Supplementary data
This investigation received financial assistance from the National Science Foundation of China (No. 21173163 and No. 21303133). Appendix A. Supplementary data
| [1] | J.H. Yi, F.Q. Zhao, W.L. Hong, et al., Effect of Bi-NTO complex on thermal behaviors, nonisothermal reaction kinetics and burning rates of NG/TEGDN/NC propellant, J. Hazard. Mater. 176 (2010) 257-261. |
| [2] | J.H. Yi, F.Q. Zhao, B.Z. Wang, et al., Thermal behaviors, nonisothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant, J. Hazard. Mater. 181 (2010) 432-439. |
| [3] | Y.L. Wang, F.Q. Zhao, J.H. Yi, New progress of study on combustion catalysts used for solid rocket propellants, Chin. J. Explos. Propell. 35 (2012) 1-7. |
| [4] | P.B. Kulkarni, G.N. Purandare, J.K. Nair, et al., Synthesis, characterization, thermolysis and performance evaluation studies on alkali metal salts of TABA and NTO, J. Hazard. Mater. 119 (2005) 53-61. |
| [5] | Q.Q. Qiu, K.Z. Xu, S.H. Yang, et al., Syntheses and characterizations of two new energetic copper-amine-DNANT complexes and their effects on thermal decomposition of RDX, J. Solid State Chem. 205 (2013) 205-210. |
| [6] | W.Q. Pang, X.Z. Fan, Y.N. Xue, et al., Study on the compatibility of tetraethylammonium decahydrodecaborate (BHN) with some energetic components and inert materials, Propell. Explos. Pyrotech. 38 (2013) 278-285. |
| [7] | R.D. Schmidt, G.S. Lee, P.F. Pagoria, A.R. Mitchell, R. Gilardi, Synthesis and properties of a new explosive 4-amino-3,5-dinitro-1H-pyrazole(LLM-116), Report UCRL-ID-148510. |
| [8] | R.D. Schmidt, G.S. Lee, P.F. Pagoria, A.R. Mitchell, R. Gilardi, Synthesis of 4-amino-3,5-dinitro-1H-pyrazole using vicarious nucleophilic substitution of hydrogen, J. Heter. Chem. 38 (2001) 1227-1230. |
| [9] | Y.L. Wang, F.Q. Zhao, Y.P. Ji, et al., Synthesis and thermal behaviors of 4-amino-3,5-dinitro-1H-pyrazole, J. Anal. Appl. Pyrol. 98 (2012) 231-235. |
| [10] | G. Hervé, C. Roussel, H. Graindorge, Selective preparation of 3,4,5-trinitro-1Hpyrazole: a stable all-carbon-nitrated arene, Angew. Chem. Int. Ed. 49 (2010) 3177-3181. |
| [11] | G. HervéVerbevie, Dinitropyrazole derivatives, their preparation, and energetic compositions comprising them, US 2009,018,693,1A1, 2009. |
| [12] | Y.Q. Zhang, A.P. Damon, M.S. Jean'ne, 4-Nitramino-3,5-dinitropyrazole-based energetic salts, Chem. Eur. J. 18 (2012) 987-994. |
| [13] | Y.L. Wang, F.Q. Zhao, K.Z. Xu, et al., Synthesis, crystal structure and thermal behavior of 4-amino-3,5-dinitropyrazole potassium salt, Inorg. Chim. Acta 405 (2013) 505-510. |
| [14] | S.H. Wu, J.H. Chi, C.C. Huang, N.K. Lin, J.J. Peng, Thermal hazard analyses and incompatible reaction evaluation of hydrogen peroxide by DSC, J. Therm. Anal. Calorim. 102 (2010) 563-568. |
| [15] | Y.L. Wang, K.Z. Xu, F.Q. Zhao, et al., Synthesis, crystal structure, and thermal behaviors of 3-nitro-1,5-bis(4,40-dimethylazide)-1,2,3-triazolyl-3-azapentane (NDTAP), Propell. Explos. Pyrotech. 38 (2013) 644-650. |
| [16] | N. Fischer, T.M. Klapötke, S. Marchner, et al., A selection of alkali and alkaline earth metal salts of 5,50-Bis(1-hydroxytetrazole) in pyrotechnic compositions, Propell. Explos. Pyrotech. 38 (2013) 448-459. |
| [17] | Y.L. Wang, F.Q. Zhao, Y.P. Ji, et al., Synthesis and thermal behaviors of 1, 8-dihydroxy-4, 5-dinitroanthraquinone barium salt, J. Anal. Appl. Pyrol. 105 (2014) 295-300. |
| [18] | K.Z. Xu, X.G. Zuo, H. Zhang, et al., Synthesis, crystal structure and thermal behavior of Cs(DNDZ), Chin. J. Inorg. Chem. 27 (2011) 2257-2262. |
| [19] | G.M. Sheldrick, SHELXTL-97, Structure Determination Software Suite, Bruker AXS, Madison, WI, 2008. |
| [20] | H.E. Kissinger, Reaction kinetics in differential thermal analysis, Anal. Chem. 29 (1957) 1702-1706. |
| [21] | T. Ozawa, A new method of analyzing thermogravimetric data, Bull. Chem. Soc. Jpn. 38 (1957) 1881-1886. |
| [22] | R.Z. Hu, S.L. Gao, F.Q. Zhao, et al., Thermal Analysis Kinetics, 2nd ed., Science Press, Beijing, 2008 (in Chinese). |