b University of Florida, 2800 SW Williston Road, Gainesville city, FL 32608, USA
Gold nanoparticles have gained increased worldwide research interest during the recent years owing to large surface-to-volume ratio and the increased surface activity [1]. Gold nanoparticles have attracted wide attention due to their potential applications in catalysis,electrical conductivity,optical properties,etc.,[2, 3, 4, 5, 6, 7]. As for the preparation of gold nanoparticles,many synthetic methods have been developed to form and to stabilize gold nanoparticles among which the reduction of chloroauric acid (HAuCl4) [8] is the most widely used,but abundant chlorine ions exist by these methods. In some areas,it can be time-consuming and expensive to remove residual chloride ions. There have been few studies on adopting other gold compounds to synthesize gold nanoparticles.
In this research,we proposed sodium gold sulfite (Na3Au(SO3)2),without chloride,to synthesize the gold nanoparticles without the introduction of other reducing reagents by using polyvinyl pyrrolidone (PVP) as a protective reagent. During the reaction process,sodium sulfite in the sodium gold sulfite molecule was converted to sodium sulfate,which has high water solubility and low adverse impact. The products formed in a few seconds to afford different shapes and sizes. The gold nanoparticles dispersed in this system have a high colloidal stability and a narrow size distribution. 2. Experimental
Chloroauric acid (HAuCl4) was prepared by my research team. Ammonia water (NH3·H2O),sodium sulfite (Na2SO3) are all guaranteed reagents purchased from Tianjin Chemical Corporation (Tianjin,China). All solutions were prepared with redistilled water. Na3Au(SO3)2 was prepared as follows: NH3·H2O was added to HAuCl4 solution,generating an orange precipitate. After repeatedly washing with redistilled water,sodium sulfite was added,and after a short time,a light yellow transparent sodium gold sulfite solution was obtained [9].
Preparation of gold nanoparticles: 3 mL of 1 g/L PVP and 40 mL of water were added to 1 mL of 0.5 mmol/L [Na3Au(SO3)2] aqueous solution,and then the mixture stirred for 1 min. Varying the temperature from 10℃ to 90℃,dilute sulfuric acid or sodium hydrate was added to regulate the solution at pH 1 ~ 9,this mixture solution was stirred for 1 min. The resulting gold nanoparticle solution was used for further characterization.
The physical characteristic of the resulting gold nanoparticles was characterized by transmission electron microscope. Samples for TEM were prepared by placing a drop of gold solution of ultrasonically dispersed product on a copper grid and then dried at room temperature. TEM images were performed using F-20 (HITACHI,Japan) operated at an accelerating voltage of 120 kV. UV-vis absorption spectra were performed by using a commercial UV-2600 spectrophotometer at room temperature with 1 cm optical path length. 3. Results and discussion
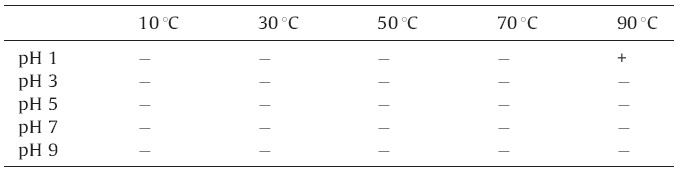
Table 1 illustrates Na3Au (SO3)2 is not reduced to atomic Au below temperature 90℃ and pH 1. In other conditions, Na3Au(SO3)2 has no obvious change in a few seconds,including the mixing solution of Na3Au(SO3)2 and PVP at 90℃,which illustrates the reducing agent is Na3Au (SO3)2 at pH 1. In this reaction process,the reaction of ionic Au(I) to atomic Au was complete,and SO32- was converted to Na2SO4. The excess of sulfate in an aqueous solution of gold nanoparticles may cause their aggregation [10],however,the concentration of the sulfate byproduct is so low that the gold nanoparticles have a good stability in solution. During the reaction,gold nanoparticles were prevented from aggregating by the dispersion affects of PVP. The reaction equation of this system is as follow:
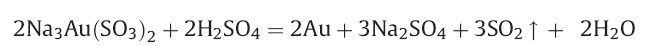
| Table 1 Oligonucleotides designed in the present study.a |
The different sizes of gold nanoparticles were obtained by using different concentrations of Na3Au(SO3)2 aqueous solution. The color of the solution changes slowly from transparent to shallow purple depending on the concentrations of the Na3Au(SO3)2 aqueous solution. This change indicates the reduction of total ionic Au(I) to atomic Au is accomplished,and gold nanoparticles were formed.
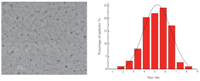
Fig. 1 illustrates the examples of the TEM micrographs and the particle size distribution for samples of gold nanoparticles prepared as described in Section 2 [9]. The surface morphology of the particles is nearly spherical and the average size of resulting particles is about 6 nm. Some previous research reported UV-vis spectrophotometry as an effective method to monitor the evolution of metal species in the synthesis of metal particles [11, 12, 13]. Fig. 1 shows the UV-vis absorption spectrum of gold nanoparticles obtained by our method. All UV-vis spectra were recorded at room temperature against the same reference sample. A strong absorption peak at approximately 520 nm indicates the formation of gold nanoparticles with an average size of particles of about 6 nm identical to the results of the TEM images. It is worthwhile mentioning that Na3Au(SO3)2 can be reduced to form gold nanoparticles,because we have not introduced other reducing reagents.
|
Download:
|
| Fig. 1.TEM images (left) and particle size distribution (right) showing characteristic of gold nanoparticles. | |
The absorption peak maximum is related to the size of the particles. The absorption spectra obtained for gold particles using different concentrations of Na3Au(SO3)2 are shown in Fig. 2. With the increase of (Na3Au(SO3)2),the synthesis of gold nanoparticles showed a different absorption peak at 524,525, 547,and 550 nm,respectively. It has been well established that the maximum wavelength of nanoparticles depends on size and shape,so this directly influences a shift of the absorption peak strongly,where larger gold particle sizes will cause an absorption peak maximum shift to longer wavelengths,lower frequency,and lower energies [14, 15, 16]. This result indicates that with the increase concentration of (Na3Au(SO3)2),the diameter of gold nanoparticles increases. Gold nanoparticles size could be controlled by adjusting the concentration of Na3Au(SO3)2. When [Na3Au(SO3)2] = 0.1 mmol/L,the gold nanoparticles have a smaller average size and a narrow size distribution.

|
Download:
|
| Fig. 2.Comparison of UV-vis spectra with concentration of 0.01 mmol/L [Na3Au (SO3)2] (left) and different concentrations of Na3Au (SO3)2 (right). | |
1. The gold nanoparticles were produced directly from a new gold
salt Na3Au(SO3)2 simply by the reduction of Au(I) with the
method of intra-molecular reduction.
2. Colloidal gold of about 6 nm particle size was prepared in
optimum conditions: T = 90℃,pH 1,[Na3Au(SO3)2] = 0.01
mmol/L,[PVP]/[Na3Au(SO3)2] = 0.3,stirring speed of 200 rpm,
the reaction time is 1 min.
3. The size of resultant gold nanoparticles can be controlled by the
concentration of gold precursor introduced.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 20976201) and the Graduate’s Scientific Research Foundation of Central South University of Forestry and Technology (No. CX2012B04).
| [1] | K. Sun, J.X. Qiu, J.W. Liu, Y.Q. Miao, Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles, J. Mater. Sci. 44 (2009) 754-758. |
| [2] | J. Xu, Q.D. Zeng, Construction of two-dimensional (2D) H-bonded supramolecular nanostructures studied by STM, Chin. Chem. Lett. 24 (2013) 177-182. |
| [3] | G.Q. Wan, D.X. Li, C.F. Li, et al., From Zn-Al layered double hydroxide to ZnO nanostructure: gradually etching by sodium hydroxide, Chin. Chem. Lett. 23 (2012) 1415-1418. |
| [4] | J. Xu, X. Han, H.L. Liu, Y. Hu, Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant, Colloids Surf. A: Physicochem. Eng. Asp. 273 (2006) 179-183. |
| [5] | Y.K. Du, P. Yang, Z.G. Mou, et al., Thermal decomposition behaviors of PVP coated on platinum nanoparticles, J. Appl. Poly. Sci. 99 (2006) 23-26. |
| [6] | G. Schmid, M. Bäumle, M. Geerkens, et al., Current and future applications of nanoclusters, Chem. Soc. Rev. 28 (1999) 179-185. |
| [7] | S. Link, Z.L. Wang, M.A. El-Sayed, Alloy formation of gold-silver nanoparticles and the dependence of the plasmon absorption on their composition, J. Phys. Chem. B 103 (1999) 3529-3533. |
| [8] | G. Frens, Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions, Nature 241 (1973) 20-22. |
| [9] | G.H. Wu, D.L. Li, K. Dong, J. Cao, Technology research about non-cyanide gold plating, Surf. Technol. 52-54 (37) (2008) 86. |
| [10] | X. Han, J. Goebl, Z. Lu, Y. Yin, Role of salt in the spontaneous assembly of charged gold nanoparticles in ethanol, Langmuir 27 (2011) 5282-5289. |
| [11] | T. del Castillo-Castro, E. Larios-Rodriguez, Z. Molina-Arenas, M.M. Castillo-Ortega, J. Tanori, Synthesis and characterization of metallic nanoparticles and their incorporation into electroconductive polymer composites, Compos. Part A: Appl. Sci. Manuf. 38 (2007) 107-113. |
| [12] | M. Shen, Y.K. Du, H.L. Rong, J.R. Li, L. Jiang, Preparation of hydrophobic gold nanoparticles with safe organic solvents by microwave irradiation method, Colloids Surf. A: Physicochem. Eng. Asp. 257-258 (2005) 439-443. |
| [13] | B. Wong, S. Yoda, S.M. Howdle, The preparation of gold nanoparticle composites using supercritical carbon dioxide, J. Supercrit. Fluids 42 (2007) 282-287. |
| [14] | Y.W. Xie, R.Q. Ye, H.L. Liu, Synthesis of silver nanoparticles in reverse micelles stabilized by natural biosurfactant, Colloids Surf. A: Physicochem. Eng. Asp. 279 (2006) 175-178. |
| [15] | M. Mandal, S. Kundu, S.K. Ghosh, T. Pal, UV-photoactivation technique for size and shape controlled synthesis and annealing of stable gold nanoparticles in micelle, Bull. Mater. Sci. 25 (2002) 509-511. |
| [16] | S. Mohapatra, N. Pramanik, S. Mukherjee, S.K. Ghosh, P. Pramanik, A simple synthesis of amine-derivatised superparamagnetic iron oxide nanoparticles for bioapplications, J. Mater. Sci. 42 (2007) 7566-7574. |