Metal catalysts have been extensively investigated because of their wide application in synthetic organic chemistry [1]. For the selective hydrogenation of chloronitrobenzene (p-CNB),noble metal catalysts such as Pd [2, 3, 4, 5],Pt [6, 7, 8, 9, 10, 11],and Ru [12, 13, 14, 15, 16, 17, 18, 19] are among the most widely investigated metal catalysts. With respect to catalytic performance,ruthenium seems to be an attractive option with its high selectivity and low cost,but its implementation is offset by low activity. Therefore,many efforts have been exerted to develop effective ruthenium catalysts in order to increase the activity while maintaining the selectivity. Many supports,such as SnO2 [16],MgF2 [17, 18, 19],and carbon nanotubes [12, 13] have been used for dispersing ruthenium nanoparticles. Despite these advances,it should be noted that the catalytic properties of the reported ruthenium catalysts were still unsatisfactory. It still remains a great challenge to develop simple, environmentally friendly,and versatile methods to fabricate ruthenium catalysts that achieve superior performance.
As a one atom-thick sheet of sp2-bonded carbon atoms, graphene has been considered as a highly promising material with special physical and chemical properties [6]. It is desirable to explore such a material as a support for catalysis. As far as we are aware,no attempt has yet been exerted to improve the catalytic properties of supported ruthenium catalysts for the selective hydrogenation of halonitroaromatics by using reduced graphene oxide (RGO) as a support. In this communication,we reported a facile synthesis of ruthenium-reduced graphene oxide composites (Ru/RGO) and investigated its catalytic properties for selective hydrogenation of p-CNB. It was found that the small Ru nanoparticles were uniformly embedded on the surface of RGO sheets with a mean size of about 2.0 nm. The Ru/RGO composites exhibited superior catalytic properties for the hydrogenation of p- CNB to the desired product. In addition,the Ru/RGO composites could be reused at least five times without loss of any activity. From a practical point of view,this study may open the way to a new approach for the synthesis of amines. 2. Experimental 2.1. Catalyst preparation
All aromatic compounds (A.R.),solvents (A.R.),and reagents (A.R.) were used as received. GO was synthesized via the improved synthesis method reported by Marcano et al. [20]. Then,0.5 g as-prepared GO was added into a flask containing 200 mL deionized water and ultrasonicated for 12 h to get a yellow-brown solution. The solution was heated to 80℃,and then 5.0 g NaBH4 was added into the flask. After 2 h,the black solid was washed with ethanol and distilled water several times and vacuum dried at 60℃ for 12 h.
The Ru/RGO composite was synthesized as follows: The asprepared RGO (0.4 g) was filled with a solution of 5.08 mg/L RuCl3 (10 mL) ultrasonicated for 3 h. Then,an aqueous solution of NaBH4 (0.3 mol/L,10 mL) was dropped into the solution to reduce Ru3+. The black solid was rinsed with ethanol and distilled water several times and vacuum dried at 60℃ for 12 h. For comparison,the active carbon (AC) supported Ru catalyst (Ru/AC) was prepared with the same procedures except that 0.4 g AC was employed as a catalyst support. 2.2. Characterization of the catalyst
The content of metal was determined by ICP (IRIS Intrepid). Transmission electron microscopy (TEM) measurements were carried out on a JEOL model 2010 instrument operated at an accelerating voltage of 200 kV. X-ray diffraction (XRD) patterns were recorded on a Rigaku X-ray diffractometer D/max-2200/PC equipped with Cu Kα radiation (40 kV,20 mA) over the range of 10-90°. X-ray photoelectron spectroscopy (XPS,Kratos XSAM800) spectra was obtained by using Al Kα radiation (12 kV and 15 mA) as an excitation source (hn = 1486.6 eV),Au (BE Au4f = 84.0 eV), and Ag (BE Ag3d = 386.3 eV) as reference. All hydrogenation samples were analyzed by gas chromatography (Agilent 7890A) with a FID detector and PEG-20M supelco column (30 m × 0.25 mm,0.25 μm film). 2.3. Activity test
Typically,to study the catalytic activity,5.0 mg of catalyst, 1.0 mmol of p-CNB,and a mixture of ethanol and water (5 mL, volume ratio of 4:1) were introduced into a 60 mL stainless steel autoclave,and the autoclave was purged with pure hydrogen five times in order to remover air. The reaction was performed with a stirring rate of 1200 rpm at 60℃ and 3.0 MPa hydrogen pressure for 2 h. 3. Results and discussion
3.1. Catalyst characterization
The content of Ru in catalyst Ru/RGO was 3.0 wt.%,as estimated by ICP. Fig. 1 shows the typical TEM images of the product of Ru/ RGO. The results demonstrate that the graphene flakes are likely to be in the form of single- or few-layer sheets,which indicates that the graphite was exfoliated to graphene flakes upon oxidation and ultrasonication [20]. Moreover,the images also show that the Ru nanoparticles are well dispersed on the surface of the RGO with a narrow particle size distribution of 1.0-2.0 nm. The average Ru particle size was estimated to 1.5 nm. The small size and even distribution of Ru nanoparticles on the surfaces of the RGO are attributed to the surface functional groups of the RGO sheets that can act as a capping material,inhibiting the aggregation of Ru clusters during reduction.

|
Download:
|
| Fig. 1.TEM images of the fresh Ru/RGO catalyst (a,b) and used five times (c,d); Ru particle size distribution of fresh Ru/RGO (e) and used five times (f). | |
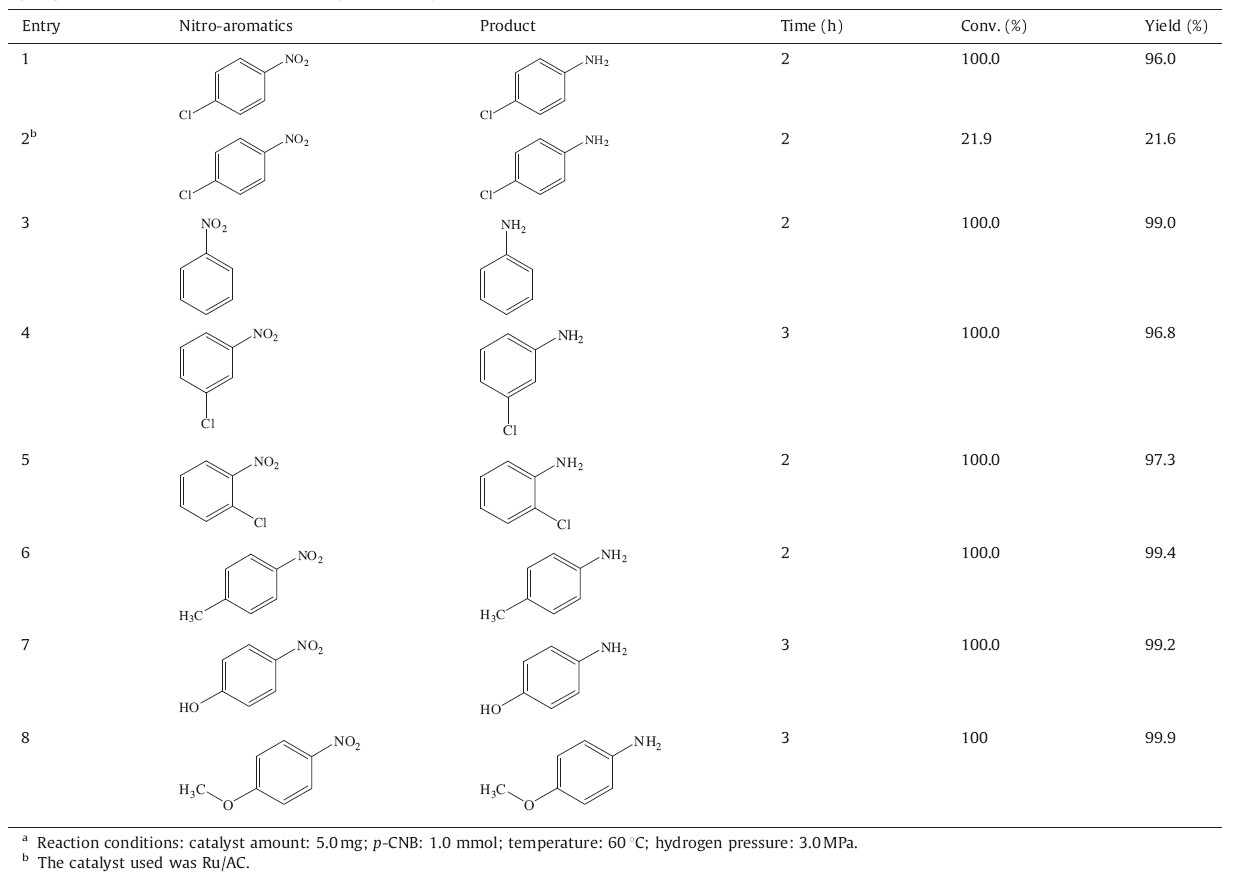
The crystal structure of Ru-RGO was also characterized by XRD in the range from 108 to 90°. For comparison,the XRD patterns of GO and RGO were also investigated and the results are shown in Fig. 2. The major diffraction lines in the powder pattern of GO comes at 2θ= 10.0° (Fig. 2a),suggesting that graphite has been successfully oxidized. When the GO was reduced by NaBH4,it can be seen that the diffraction peaks at 10.0° disappeared and two new peaks appeared at 25.4 and 43.1° (see inset of Fig. 2) corresponding to (0 0 2) and (1 0 0) reflection of RGO [6], respectively,indicating that GO was successfully reduced using NaBH4 as a reducing agent. Moreover,no obvious changes were observed after depositing Ru nanoparticles on the surface of the RGO,and the corresponding diffraction peaks of Ru were not observed in the as-synthesized composite,demonstrating that the Ru nanoparticles are quite small and well-dispersed on the surface of RGO,which agrees well with the TEM results.
|
Download:
|
| Fig. 2.XRD patterns of (a) GO; (b) RGO; (c) Ru/RGO. Inset is the amplified pattern of RGO and Ru/RGO. | |
The XPS elemental survey scans of the surface of the Ru-RGO composites show that the peaks corresponding to oxygen,carbon, and ruthenium are distinctly detected. In order to evaluate the electronic state of Ru in the Ru-RGO catalyst,the binding energy of Ru was also determined. Because of the partial overlap between the C1s and Ru3d peaks,which prevents the accurate determination of the Ru species,Ru3p spectrum was characterized to distinguish the electronic state of Ru. As shown in Fig. 3a,the binding energy of Ru3p level in Ru/RGO catalyst is 463.6 eV,which is higher than that of standard zero-valent state Ru (462.9 eV),indicating that there is a strong metal-support interaction [21, 22]. In addition,The C1s spectra of RGO (Fig. 3b) could be deconvoluted into four peaks at 284.6,285.8,287.7,and 289.6 eV,which are associated with C-C, C-OH,C(epoxy/alkoxy),and C55O groups,respectively [23].

|
Download:
|
| Fig. 3.XPS spectrum of catalyst Ru/RGO (a) Ru3p; (b) Ru3d+C1s. | |
The catalytic properties of the Ru/RGO catalyst were investigated using the selective hydrogenation of p-CNB as a model reaction and the results are presented in Table 1. The conversion of p-CNB was negligible without catalyst,which shows that the presence of Ru is indispensable for high catalytic activity. The main product was p-CAN; aniline (AN) and p-ClPhNHC2H5 were detected as by-products in the hydrogenation process. It can be seen that the Ru/RGO catalyst was extremely catalytically active for p-CNB hydrogenation in ethanol-water mixture. The conversion of p-CNB achieved 100% yield with a selectivity of 96% towards p-CAN at 60℃ and a hydrogen pressure of 3.0 MPa in 2 h,while the conversion of Ru/AC was only 21.9% with a comparable selectivity of Ru/RGO at the same reaction conditions.
Wealso compared the hydrogenation of CNBs over the Ru based catalysts reported recently. For example,Zuo et al. [16] developed the synthesis of Ru/SnO2 nanoparticles,which exhibited a turnover frequency number of 155 h-1 with a selectivity of 99.9% to o-CAN at 60℃ and 4.0 MPa H2. Pietrowski et al. [17] reported that the Ru/ MgF2 catalyst exhibited high catalytic activity with a TOF of 1267 h-1 with a selectivity of 97% at 353 K and 4.0 MPa H2. Oubenali et al. [13] prepared carbon nanofibers (CNFs) and carbon nanotubes (CNTs) supported ruthenium catalysts for the selective hydrogenation of p-CNB at 60℃ and 3.5 MPa H2. Although,a high TOF value of 1898 h-1 was observed,they can only get a selectivity of 92-94% towards p-CAN. The present work reports the use of RGO as a support for the deposition of Ru for the selective hydrogenation of p-CNB. A selectivity of 96% towards p-CAN coexisting with a TOF value of 420 h-1 was obtained at relatively low temperature and hydrogen pressure. The good catalytic performance of Ru/RGO for the selective hydrogenation p-CNB is probably attributed to the narrow mean particle size of Ru (1.5 nm) and the high dispersion of Ru nanoparticles on the surface of the RGO sheets via reduction of Ru3+ by aqueous NaBH4. The high dispersion of Ru on the surface of RGO would offer more active sites for the hydrogenation,which increase the adsorption probability of reactant molecules for speeding up the reaction.
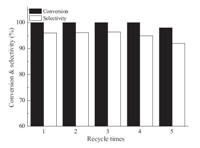
Furthermore,we also examined the catalytic properties of Ru/ RGO for other nitroarenes. As shown in Table 1,the catalyst showed good catalytic performance in the investigated substrates containing electron-donating groups or electron-withdrawing groups. For example,chloronitrobenzenes can be quickly hydrogenated to the desired product. Moreover,the aryl nitro containing electron-donating substituents,such as -OH,-CH3,and -OCH3 also can be easily reduced with extremely high yield (>99%). The stability of the Ru/RGO catalyst was also studied using p-CNB as substrate under the same conditions. The catalyst was separated from the reaction system by centrifugation and the supernatant liquid was removed. The black solid was thoroughly washed three times with ethanol,dried in vacuum,and reused for the next run.
| Table 1 Hydrogenation of different substrates over Ru/RGO and Ru/ACa. |
|
Download:
|
| Fig. 4.Reusability of the catalyst Ru/RGO. | |
In summary,a facile synthesis of Ru/RGO nano-catalyst has been established. This catalyst shows extremely superior catalytic properties for the selective hydrogenation of p-CNB to p-CAN and can be reused at least five times. The excellent performance of the Ru/RGO catalyst is attributed to the small size of Ru nanoparticles and the even dispersion of the Ru nanoparticles on the surface of the RGO sheet. Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21207109),Scientific Research Fund of Sichuan Provincial Education Department (No. 11ZA034), and the Opening Project of Key Laboratory of Green Catalysis of Sichuan Institutes of High Education (No. LZJ1205).
| [1] | J. Zhang, Y. Wang, H. Ji, et al., Magnetic nanocomposite catalysts with high activity and selectivity for selective hydrogenation of ortho-chloronitrobenzene, J. Catal. 229 (2005) 114-118. |
| [2] | F.W. Zhang, J. Jin, X. Zhong, et al., Pd immobilized on amine-functionalized magnetite nanoparticles: a novel and highly active catalyst for hydrogenation and Heck reactions, Green Chem. 13 (2011) 1238-1243. |
| [3] | C. Liang, J. Han, K. Shen, et al., Palladium nanoparticle microemulsions: formation and use in catalytic hydrogenation of o-chloronitrobenzene, Chem. Eng. J. 165 (2010) 709-713. |
| [4] | Q. Xu, X.M. Liu, J.R. Chen, R.X. Li, X.J. Li, Modification mechanism of Sn4+ for hydrogenation of p-chloronitrobenzene over PVP-Pd/g-Al2O3, J. Mol. Catal. A: Chem 260 (2006) 299-305. |
| [5] | C. Su, X.N. Li, Q.F. Zhang, et al., Behavior of adsorbed diphenyl-sulfide on the Pd/C catalyst for o-chloronitrobenzene hydrogenation, Chin. Chem. Lett. 24 (2013) 59-62. |
| [6] | R. Nie, J. Wang, L. Wang, et al., Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes, Carbon 50 (2012) 586-596. |
| [7] | Y. Motoyama, Y. Lee, K. Tsuji, et al., Platinum nanoparticles supported on nitrogendoped carbon nanofibers as efficient poisoning catalysts for the hydrogenation of nitroarenes, ChemCatChem 3 (2011) 1578-1581. |
| [8] | K.L. Xu, Y. Zhang, X.R. Chen, et al., Convenient and selective hydrogenation of nitro aromatics with a platinum nanocatalyst under ambient pressure, Adv. Synth. Catal. 353 (2011) 1260-1264. |
| [9] | Z. Sun, Y. Zhao, Y. Xie, et al., The solvent-free selective hydrogenation of nitrobenzene to aniline: an unexpected catalytic activity of ultrafine Pt nanoparticles deposited on carbon nanotubes, Green Chem. 12 (2010) 1007-1011. |
| [10] | W.X. Tu, S.J. Cao, L.P. Yang, W.C. Wang, Modification effects of magnetic supports and bimetallic structures on palladium nanocluster catalysts, Chem. Eng. J. 143 (2008) 244-248. |
| [11] | Y. Motoyama, K. Kαmo, H. Nagashima, Catalysis in polysiloxane gels: platinumcatalyzed hydrosilylation of polymethylhydrosiloxane leading to reusable catalysts for reduction of nitroarenes, Org. Lett. 11 (2009) 1345-1348. |
| [12] | C. Antonetti, M. Oubenali, A.M.R. Galletti, P. Serp, G. Vannucci, Novel microwave synthesis of ruthenium nanoparticles supported on carbon nanotubes active in the selective hydrogenation of p-chloronitrobenzene to p-chloroaniline, Appl. Catal. A 421 (2012) 99-107. |
| [13] | M. Oubenali, G. Vanucci, B. Machado, et al., Hydrogenation of p-chloronitrobenzene over nanostructured-carbon-supported ruthenium catalysts, ChemSusChem 4 (2011) 950-956. |
| [14] | J. Ning, J. Xu, J. Liu, et al., A remarkable promoting effect of water addition on selective hydrogenation of p-chloronitrobenzene in ethanol, Catal. Commun. 8 (2007) 1763-1766. |
| [15] | M. Liu, W. Yu, H. Liu, Selective hydrogenation of o-chloronitrobenzene over polymer-stabilized ruthenium colloidal catalysts, J. Mol. Catal. A: Chem. 138 (1999) 295-303. |
| [16] | B. Zuo, Y. Wang, Q. Wang, et al., An efficient ruthenium catalyst for selective hydrogenation of ortho-chloronitrobenzene prepared via assembling ruthenium and tin oxide nanoparticles, J. Catal. 222 (2004) 493-498. |
| [17] | M. Pietrowski, M. Zieliński, M. Wojciechowska, Selective reduction of chloronitrobenzene to chloroaniline on Ru/MgF2 catalysts, Catal. Lett. 128 (2009) 31-35. |
| [18] | M. Pietrowski, M. Zieliński, M. Wojciechowska, High-selectivity hydrogenation of chloronitrobenzene to chloroaniline over magnesium fluoride-supported bimetallic ruthenium-copper catalysts, Chem. Cat. Chem. 3 (2011) 835-838. |
| [19] | M. Pietrowski, M. Wojciechowska, An efficient ruthenium-vanadium catalyst for selective hydrogenation of ortho-chloronitrobenzene, Catal. Today 142 (2009) 211-214. |
| [20] | D.C. Marcano, D.V. Kosynkin, J.M. Berlin, et al., Improved synthesis of graphene oxide, ACS Nano 4 (2010) 4806-4814. |
| [21] | X. Xu, X. Li, H. Gu, Z. Huang, X. Yan, A highly active and chemoselective assembled Pt/C (Fe) catalyst for hydrogenation of o-chloronitrobenzene, Appl. Catal. A 429- 430 (2012) 17-23. |
| [22] | J.B. Goodenough, R. Manoharan, A. Shukla, K.V. Ramesh, Intraalloy electron transfer and catalyst performance: a spectroscopic and electrochemical study, Chem. Mater. 1 (1989) 391-398. |
| [23] | S. Liu, J. Tian, L. Wang, et al., Self-assembled graphene platelet-glucose oxidase nanostructures for glucose biosensing, Biosens. Bioelectron. 26 (2011) 4491-4496. |