The forming of C-C bonds is one of the most important tasks in organic synthesis. The development of transition metal-catalyzed coupling reactions greatly improves the efficiency of the building of C-C bonds in recent decades [1]. However,some of the methods need pre-functionalized materials [2]. Li proposed the concept of Cross-dehydrogenative-coupling (CDC) reactions,which combine two C-H bonds to form a new C-C bond,avoiding the need for preparing pre-functionalized materials [3]. Then,Cu-catalysts [4], Fe-catalysts [5],Pd-catalysts [6] and other metal catalysts for CDC reactions were successively reported [7, 8]. However,the development of metal free CDC reactions has great significance in bulk products and the production of fine chemicals [3].
The phenanthrene ring is an important unit of tylophora alkaloids. These alkaloids have polycyclic structures and several bioactivities,especially antitumor activity,which aroused great interest among chemists and pharmaceutical scientists [9, 10]. The key step of constructing such alkaloids and their analogs is the preparation of polymethoxy-substituted phenanthrene [11]. Therefore,a simple and efficient method for such phenanthrenes is noteworthy. Number of synthetic methods have been proposed for this CDC reaction,such as Pb(OAc)4,VOF3,VOCl3,FeCl3 [12]. The application of these methods was restricted by tedious linear procedures,low overall yields or difficult purification procedures. Thus,it is necessary to find new metal free methods to broaden the choices of building phenanthrenes.
Herein,di-tert-butylperoxide (DTBP) was reported for the intramolecular oxidative coupling reaction of unfunctionalized 2,3-diphenylacrylic acids and their derivatives at room temperature in trifluoroacetic acid (TFA)/CH3CN to give polysubstituted phenanthrenes in good to excellent yields. 2. Experimental
All reactions were performed under air atmosphere unless stated otherwise. DTBP was purchased from the Shanghai Taitan Technology Co.,Ltd. TFA was provided by Shanghai Darui Finechem Ltd. All solvents,such as dichloromethane,acetonitrile and toluene,were purchased from Kelong Chemical Reagent (Chengdu, China) and were dried and purified by standard techniques before use. 1H NMR and 13C NMR spectra were recorded using a Varian Mercury Plus 400 and a Bruker Avance 600 spectrometer. HRMS data were recorded on a BioTOFQ mass spectrometer (ESIMS). The melting points were determined on an X-4 binocular microscope melting point apparatus (Shanghai Precision Tech Instruments Co., Shanghai,China) and were uncorrected. The Supporting information includes detail experimental procedures and the spectra data of all substrates and products. 2.1. General procedure for the preparation of 2,3-disubstituted phenylacrylic acid
A mixture of homoveratric acid,veratraldehyde,acetic anhydride and triethylamine was stirred and heated at reflux for 24 h under nitrogen atmosphere. The products were purified and collected to give (E)-2,3-bis(3,4-dimethoxyphenyl)acrylic acid (1a) and (Z)-2,3-bis(3,4-dimethoxyphenyl)acrylic acid (1b) [13, 14]. By following the same procedure as above (E)-2-(3,4-dimethoxyphenyl)- 3-(3,4-methylenedioxyphenyl)acrylic acid (1c) and (Z)-2- (3,4-dimethoxyphenyl)-3-(3,4-methylenedioxyphenyl)acrylic acid (1d) were obtained. 2.2. General procedure for the preparation of methyl 2,3-disubstituted phenylacrylate
Esterification of 1a in methanol and sulfuric acid in the usual manner followed by a recrystallization from ethyl acetate afforded the pure methyl (E)-2,3-bis(3,4-dimethoxyphenyl)acrylate (1e) as a white solid. Using the same procedures,(Z)-2,3-bis(3,4- dimethoxyphenyl)acrylate (1f),methyl (E)-2-(3,4-dimethoxyphenyl)- 3-(3,4-methylenedioxyphenyl)acrylate (1g),methyl (Z)-2- (3,4-dimethoxyphenyl)-3-(3,4-methylenedioxyphenyl)acrylate (1h) were also obtained [15]. 2.3. Preparation of (Z)-2,3-bis(3,4-dimethoxyphenyl)acrylonitrile(1i)
A mixture of cyanide,veratraldehyde,ethanol,and sodium ethoxide was stirred and heated to reflux for 2 h. The product was collected and dried to give 1i as a yellow solid [16]. 2.4. General procedures for the oxidative coupling reaction
To a solution of 1e (0.2 mmol,71.6 mg) in acetonitrile (2 mL) was added DTBP (0.2 mmol × 2 mmol,58.4 mg) and CF3COOH (4 mL). The mixture was stirred and the reaction tube was sealed at room temperature for 4 h and then quenched by water (10 mL). The aqueous phase was extracted with dichloromethane (3 mL × 10 mL). The combined organic extracts were dried over with MgSO4,filtered,concentrated using a rotary evaporator and purified by chromatographic column to give the desired product 2c as a light yellow solid. Coupling reactions of the other substrates followed the same procedure.
Methyl 2,3,6,7-tetramethoxyphenanthrene-9-carboxylate (2c): Yield 88.5%. mp 203-204℃ [15]; 1H NMR (400 MHz,CDCl3): δ 8.65 s,1H),8.42 (s,1H),7.80 (s,1H),7.76 (s,1H),7.26 (s,1H),4.14 (s, 3H),4.13 (s,3H),4.08 (s,3H),4.04 (s,3H),4.02 (s,3H).
2,3,6,7-Tetramethoxyphenanthrene-9-carboxylic acid (2a): Yield 91.4%. mp 283-285℃ [13]; 1H NMR (400 MHz,DMSO): δ 12.86 (brs,1H,-COOH),8.55 (s,1H),8.42 (s,1H,H-30),8.05 (s,1H), 8.01 (s,1H),7.56 (s,1H),4.06 (s,3H),4.05 (s,3H),3.92 (s,3H),3.89 (s,3H).
2,3-Methylenedioxy-6,7-dimethoxyphenanthrene-9-carboxylate acid (2b): Yield 86.4%. mp 265-266℃ [14]; 1H NMR (400 MHz, CDCl3): δ 8.60 (s,1H),8.34 (s,1H),7.80 (s,1H),7.73 (s,1H),7.22 (s, 1H),6.12 (s,2H),4.09 (s,3H),4.07 (s,3H),4.01 (s,3H).
Methyl 2,3-methylenedioxy-6,7-dimethoxyphenanthrene-9- carboxylic (2d): Yield 92.3%. mp. 210-213℃; 1H NMR (400 MHz,CDCl3): δ 8.60 (s,1H),8.34 (s,1H),7.80 (s,1H),7.73 (s,1H),7.22 (s,1H),6.12 (s,2H),4.09 (s,3H),4.07 (s,3H),4.01 (s, 3H). 13C NMR (100 MHz,CDCl3): δ 168.1,149.9,149.2,148.9,147.1, 130.5,128.7,125.6,125.4,124.1,122.1,106.5,106.4,102.5,101.5,100.2,55.9,55.8,52.1. IR (KBr,cm-1): ν 2997,2947,2834,1706, 1595,1500,1434,1348,1257,1257,1136,1034; HRMS (ESI): Calcd. for C19H16O6Na: m/z 363.0839 ([M+Na]+),found: 363.0841.
2,3,6,7-Tetramethoxyphenanthrene-9-carbonitrile (2e): Yield 85.1%. mp 266-268℃ [16]; 1H NMR (400 MHz,CDCl3): δ 7.99 (s, 1H),7.73 (s,1H),7.71 (s,1H),7.50 (s,1H),7.18 (s,1H),4.15 (s,3H), 4.14 (s,3H),4.08 (s,3H),4.05 (s,3H). 3. Results and discussion
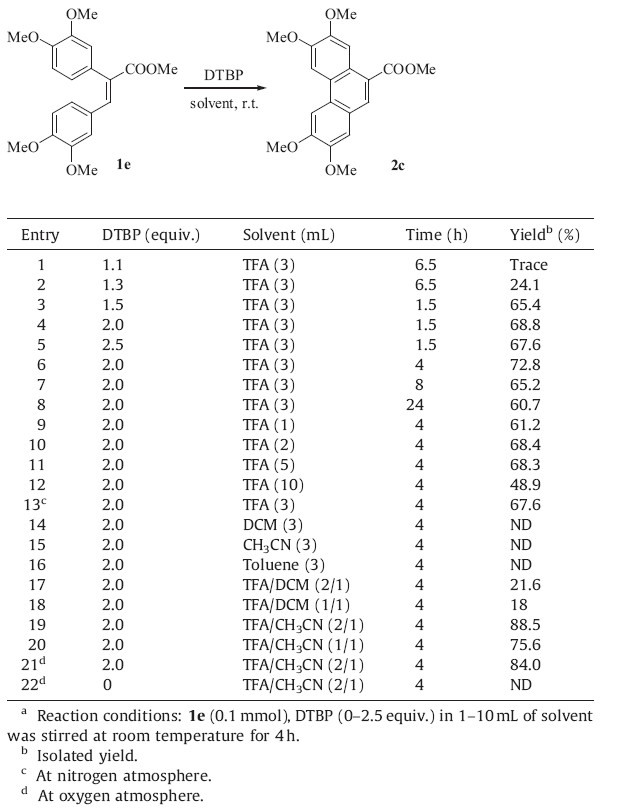
(E)-Methyl 2,3-bis(3,4-dimethoxyphenyl)acrylate (1e) was used as the substrate to investigate proper reaction conditions for the desired coupling reaction. The results were summarized in Table 1. To investigate the influence of DTBP,different quantities were added and the desired product 2c was obtained in up to 68.8% yield (Table 1,entries 1-5). Unreacted 1e existed after 1.5 h when 2.0 equiv. DTBP was added. However,when the reaction time was prolonged to 4 h,the yield improved but 1e still did not react completely (Table 1,entry 6). Further extension of the reaction time to 8 h and 24 h did not improve the yield (Table 1,entries 7 and 8). Then,with 2.0 equiv. of DTBP and reaction time unchanged, changing the dosage of TFA (1 mL,2 mL,3 mL,5 mL,10 mL),3 mL TFA brought a better yield of 72.8% (Table 1,entries 6,9,10,11,12). Under the nitrogen atmosphere,the result was not improved (Table 1,entry 13). Literatures reports suggested that CH2Cl2 was frequently used in the CDC reactions such as the transformation of 1e-2c [12]. For this method,however,TFA was more suitable than CH2Cl2 (Table 1,entries 8,14).
| Table 1 Optimization of the intramolecular coupling reaction.a |
The coupling reaction of 1e-2c could proceed in moderate yield when substrate 1e (0.1 mmol) was reacted with 2.0 equiv. of DTBP and 3 mL of TFA for 4 h. The amount of DTBP and TFA or the reaction time has no significant impact on the yield.
The common solvents such as CH2Cl2,CH3CN or toluene do not afford the desired product 2c (Table 1,entries 14,15,16). And the yields were not satisfactory when mixed solvents of CH2Cl2 and TFA were used (Table 1,entries 17 and 18). When TFA/CH3CN (2/1, v/v) were used,the isolated yields increased from 72.8% to 88.5% (Table 1,entries 6 and 19). Even the concentration of TFA was decreased,we could achieve 75.6% yield (Table 1,entry 20). Under the oxygen atmosphere,the yield did not improve (Table 1,entries 19 and 21). When oxygen was the sole oxidant,the reaction did not occur (Table 1,entry 22).
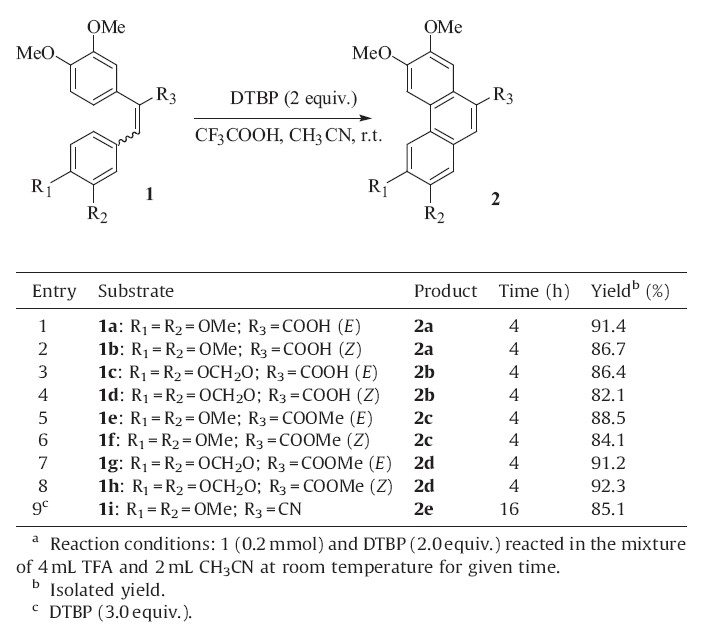
The optimized reaction conditions were summarized as following: A mixture of 2.0 equiv. of DTBP and 0.1 mmol of the substrate in TFA/CH3CN (2/1,v/v) was stirred at room temperature for 4 h. Various 1,2-diaryethylene derivatives were successfully converted to phenanthrenes under these optimized conditions (Table 2).
| Table 2 Substrate scope of intramolecular oxidative coupling with DTBP as sole oxidant.a |
In summary,a metal free intramolecular oxidative coupling reaction was developed with di-tert-butylperoxide as the sole oxidant at room temperature in TFA/CH3CN to yield polymethoxysubstituted phenanthrenes in good to excellent yields. Acknowledgments
The authors are grateful to Sichuan Provincial Education Department (No. 12ZA141) and Key Laboratory of Advanced Functional Materials of Sichuan Province Higher Education System (No. KFKT2013-01) and Sichuan Normal University (No. XYZ2013- 14-37) for financial support. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013. 11.004.
| [1] | (a) V. Ritleng, C. Sirlin, M. Pfeffer, Ru-, Rh-, and Pd-catalyzed C-C bond formation involving C-H activation and addition on unsaturated substrates: reactions and mechanistic aspects, Chem. Rev. 102 (2002) 1731-1770; (b) Y.Q. He, N.N. Zhang, Y. Liu, et al., Facile synthesis and excellent catalytic activity of gold nanoparticles on graphene oxide, Chin. Chem. Lett. 23 (2012) 41- 44; (c) W.P. Mai, H.H. Wang, J.W. Yuan, L.R. Yang, Z.C. Li, Palladium-catalyzed suzuki couplings using a novel diaminophosphine oxide as ligand, Chin. Chem. Lett. 23 (2012) 521-524. |
| [2] | (a) T. Naota, H. Takaya, S.I. Muragashi, Ruthenium-catalyzed reactions for organic synthesis, Chem. Rev. 98 (1998) 2599-2660; (b) D.F. Wu, M.J. Yang, Y. Wang, G.W. Gao, J. Men, A facile and efficient synthetic method for 4-phenylethynylphthalic anhydride, Chin. Chem. Lett. 22 (2011) 159- 162. |
| [3] | X.W. Guo, Z.P. Li, C.J. Li, Cross-dehydrogenative-coupling (CDC) reaction, Prog. Chem. 22 (2010) 1434-1441. |
| [4] | Z.P. Li, C.J. Li, CuBr-catalyzed efficient alkynylation of sp3 C-H bonds adjacent to a nitrogen atom, J. Am. Chem. Soc. 126 (2004) 11810-11811. |
| [5] | (a) C. Bolm, J. Legros, J. Le Pail, L. Zani, Iron-catalyzed reactions in organic synthesis, Chem. Rev. 104 (2004) 6217-6254; (b) Y. Song, X.S. Tang, X.M. Hou, Y.J. Bai, Advances of iron(Ⅲ) chloride-catalyzed organic reactions, Chin. J. Org. Chem. 33 (2013) 76-89. |
| [6] | K.L. Hull, E.L. Lanni, M.S. Sanford, Highly regioselective catalytic oxidative coupling reactions: synthetic and mechanistic investigations, J. Am. Chem. Soc. 128 (2006) 14047-14049. |
| [7] | G.J. Deng, C.J. Li, Sc(OTf)3-catalyzed direct alkylation of quinolines and pyridines with alkanes, Org. Lett. 11 (2009) 1171-1174. |
| [8] | A. Sud, D. Sureshkumar, M. Klussmann, Oxidative coupling of amines and ketones by combined vanadium- and organocatalysis, Chem. Commun. (2009) 3169- 3171. |
| [9] | Z.G. Li, Z. Jin, R.Q. Huang, Isolation, total synthesis and biological activity of phenanthroindolizidine and phenanthroquinolizidine alkaloids, Synthesis (2001) 2365-2378. |
| [10] | C.G. Zhang, J.J. Li, X.H. Wang, C. Feng, B.Q. Wang, Progress on relationship of tylophora alkaloids and their antitumor activity, Chin. J. Med. Chem. 20 (2010) 379-388. |
| [11] | (a) K. Kim, T. Lee, E. Lee, et al., Asymmetric total syntheses of (-)-antofine and (-)- cryptopleurine using (R)-(E)-4-(tributylstannyl)but-3-en-2-ol, J. Org. Chem. 69 (2004) 3144-3149; (b) A. Camacho-Davila, J.W. Herndon, Total synthesis of antofine using the net [5 + 5]-cycloaddition of γ,δ-unsaturated carbene complexes and 2-alkynylphenyl ketones as a key step, J. Org. Chem. 71 (2006) 6682-6685; (c) K. Kim, Y.M. Lee, J. Lee, et al., Expedient syntheses of antofine and cryptopleurine via intramolecular 1,3-dipolar cycloaddition, J. Org. Chem. 72 (2007) 4886-4891. |
| [12] | (a) D.A. Evans, C.J. Dinsmore, D.A. Evrard, K.M. Devries, Oxidative coupling of arylglycine-containing peptides. A biomimetic approach to the synthesis of the macrocyclic actinoidinic-containing vancomycin subunit, J. Am. Chem. Soc. 115 (1993) 6426-6427; (b) E.C. Taylor, J.G. Andrade, G.J.H. Rall, A. Mckillop, Thallium in organic synthesis. 59. Alkaloid synthesis via intramolecular nonphenolic oxidative coupling. Preparation of (±)-ocoteine, (±)-acetoxyocoxylonine, (±)-3-methoxy-n-acetylnornantenine, (±)-neolitsine, (±)-kreysigine, (±)-O-methylkreysigine, and (±)- multifloramine, J. Am. Chem. Soc. 102 (1980) 6513-6519; (c) K.L. Wang, M.Y. Lü, Q.M. Wang, R.Q. Huang, Iron(Ⅲ) chloride-based mild synthesis of phenanthrene and its application to total synthesis of phenanthroindolizidine alkaloids, Tetrahedron 64 (2008) 7504-7510. |
| [13] | D.R. Ji, L.D. Su, C.G. Zhang, Intramolecular oxidative coupling reaction of 4- phenylmethyl-ene-3-isochromanones with 2,3-dichloro-5,6-dicyanobenzo-quinone as an oxidant, Chin. J. Org. Chem. 32 (2012) 2334-2338. |
| [14] | Z.W. Wang, M. Wu, Y. Wang, et al., Synthesis and SAR studies of phenanthroindolizidine and phenanthroquinolizidine alkaloids as potent anti-tumor agents, Eur. J. Med. Chem. 51 (2012) 250-258. |
| [15] | K.L. Wang, M.Y. Lü, A. Yu, X.Q. Zhu, Q.M. Wang, Iron(Ⅲ) chloride catalyzed oxidative coupling of aromatic nuclei, J. Org. Chem. 74 (2009) 935-938. |
| [16] | T.F. Buckley Ⅲ, R. Henry, Amino acids as chiral educts for asymmetric products. Chirally specific syntheses of tylophorine and cryptopleurine, J. Org. Chem. 48 (1983) 4222-4232. |