b School of Biological and Chemical Engineering, Jiaxing University, Jiaxing 314001, China
Carbon-oxygen bonds extensively exist in all kinds of biologically active natural products,important pharmaceutical compounds and polymers [1, 2]. The palladium-catalyzed formation of carbon-oxygen bonds is one of the two major methods available for aryl ether synthesis. However,palladium-based protocols,although successful [3],have some inherent limitations such as moisture sensitivity,costly metal catalysts,and environmental toxicity. These shortcomings limit their large scale applications in industrial fields,particularly in the pharmaceutical industry,where metal contamination to the products may cause serious problems. The other method for ether syntheses is the copper-mediated Ullmann reaction. The harsh classical conditions of the Ullmann ether synthesis also have limitations in its application because of the high temperatures (120-220℃) required and the use of stoichiometric amounts of copper reagents [4]. In the past years,significant advances have been achieved for the copper-catalyzed synthesis of diaryl ethers using new ligands such as 1-naphthoic acid [5a],8-hydroxyquinoline [5b,5c], 2,2,6,6-tetramethylheptane-3,5-dione [5d],amino acids [5e,5f,5g], diimine ligands [5h,5i],PPAMP [5j],phosphazene P4-t-Bu base [5k],β-ketoester [5l],1,1'-binaphthyl-2,2'-diamine [5m],tripod ligands [5n],silica supported Cu(II) [5o],aryl boronic acid [5p],tetraethyl orthosilicate (as solvent) [5q],(2-pyridyl)acetone [5r], nBu4NBr (as a phase transfer catalyst) [5s],picolinic acid [5t], methenamine [5u] and some copper(I) complexes [5v,5w,5x]. With the carefully selected combinations of a catalytic amount of copper sources,bases,and supporting ligands,aryl bromides and aryl iodides had been reported to couple with phenols with excellent yields under mild conditions. Therefore,further discovery of new facile ligands for the copper-catalyzed crosscoupling reactions of phenols with aryl halides is still an area of considerable interest.
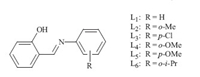
Salicylaldimine ligands are a type of simple and versatile bidentate ligand,and they can be readily prepared by the condensation of salicylaldehyde with amines. Moreover,the steric and electronic properties of these ligands can be easily tuned using appropriate substituents. These ligand systems have been utilized in main group,transition-metal and lanthanides coordination chemistry,and some of these metal complexes show good activity in homogeneous catalysis [6]. To our knowledge,there are very few examples available in the copper-catalyzed arylation of phenols involving these ligands. Herein we wish to report the efficient use of salicylaldimine ligands (Fig. 1) for the copper catalyzed Oarylation of phenols with aryl halides.
|
Download:
|
| Fig. 1.Structures of ligand. | |
2-((2-Isopropylphenylimino)methyl)phenol (15 mol%),CuI (5 mol%),phenol (1.5 mmol,141 mg) and K3PO4 (2.0 mmol, 425 mg) were added to a screw-capped Schlenk tube under argon.The tube was then evacuated and backfilled with argon (three cycles). Iodobenzene (1 mmol,0.11 mL) and dry dioxane (0.5 mL) were added by syringe at room temperature. The reaction mixture was stirred at needed temperature (101℃) for 24 h. The reaction mixture was allowed to reach room temperature and then diluted with dichloromethane (10 mL). The slurry was filtered,and the filter cake was washed with 10 mL of dichloromethane. The solvent was removed in vacuo,and the residue was purified by column chromatography on silica gel to afford the desired product (3aa). 3. Results and discussion
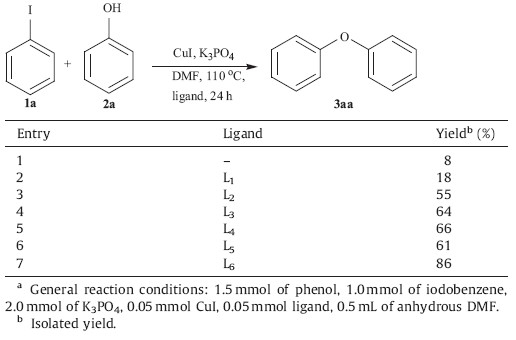
The ligands L1-L6 were synthesized by known literature methods (Fig. 1) [7]. These ligands (L1-L6) are examined using iodobenzene and phenol as model substrates in the presence of tripotassium phosphate and copper salts at 110℃ for 24 h in DMF. The results are listed in Table 1. The desired product was obtained in 8% yields in the absence of the ligand (Table 1,entry 1). Among the ligands L1-L6 tested,L6 is most efficient (Table 1,entries 2-7). This might be due to the suitable steric hindrance of L6.
| Table 1 Comparison of various ligands in the coupling reaction.a |
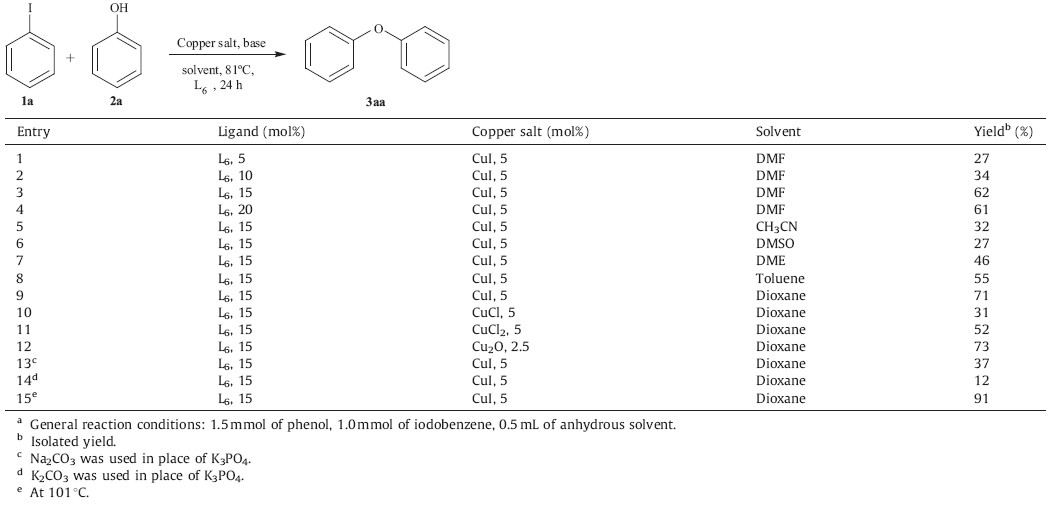
Subsequently,L6 was selected as the ligand for assisting Cu salts in catalyzing O-arylation of phenols with aryl halides to further investigate the effects of the other conditions on the O-arylation, including alkalis,solvents,copper salt,etc. The results are listed in Table 2. The results showed the optimal ratio of Cu(I) (5 mmol%) to L6 used in this study is 1:3 (Table 2,entries 1-4). Then,on turning our attention to examining the solvent effects at 81℃,we find that dioxane as a solvent gave the best result,furnishing diphenyl ether in 71% yield. DMF,DME,THF,DMSO,toluene and CH3CN were not as good as dioxane (Table 2,entries 3 and 5-9). For comparison on the efficiency of copper salts in the O-arylation reactions,both Cu2O and CuI were found to be highly effective,while CuCl2 and CuCl were moderate (Table 2,entries 9-12). After screening a series of bases,2 equiv. of K3PO4 was determined to be most effective and gave diphenyl ether in 71% yield at 81℃ (Table 2, entries 9 vs. 13 and 14). To further optimize the reaction conditions,increased yields of the coupling products were observed by simply increasing the reaction temperature to 101℃ (91%,Table 2,entry 15). As previously reported [4],a slight excess of the phenol was employed (0.5 equiv. excess) since the use of 1.0 equiv. resulted in very slow conversion as the reaction neared completion. Therefore,the combination of CuI (5%),15% L6 and 200% K3PO4 in dioxane at 101℃ was chosen as the optimal conditions for further exploration.
| Table 2 Copper-catalyzed O-arylation of phenol with aryl halide: optimization of the reaction conditions.a |
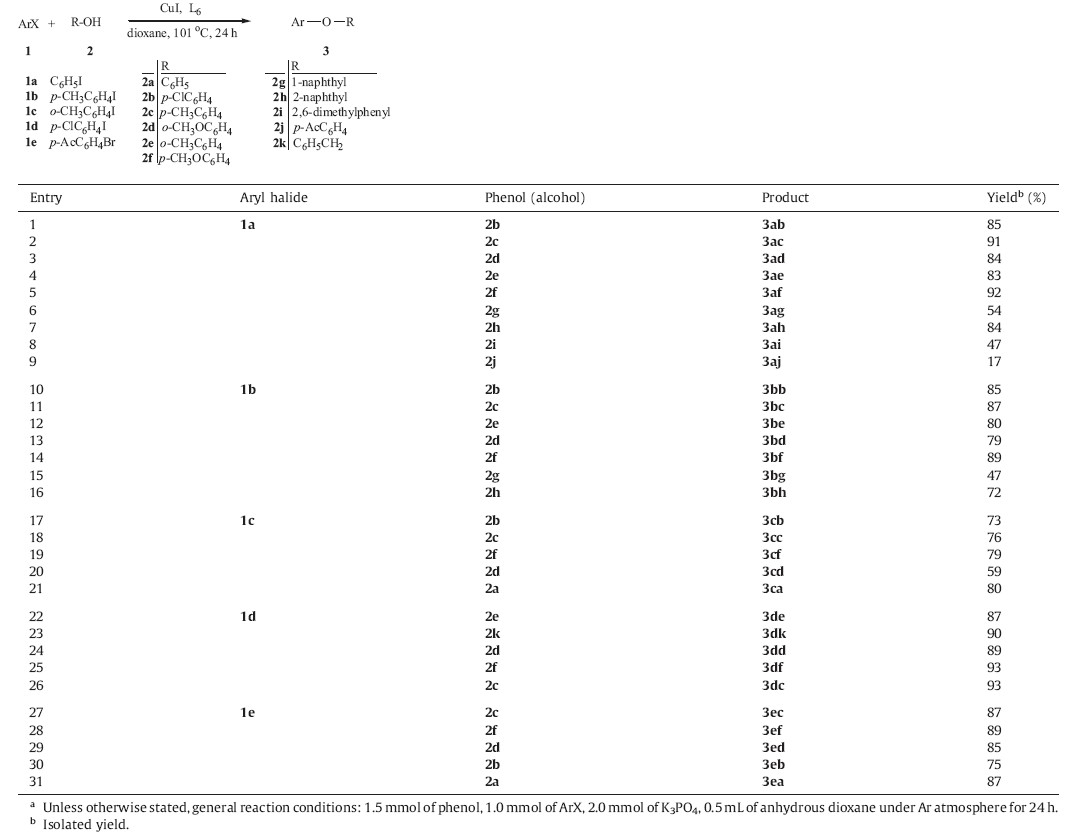
The scope of the copper-catalyzed C-O bond formation was explored by using a variety of aryl iodides (or bromides) with substituted phenols under the optimized conditions. As shown in Table 3,O-arylation of aryl iodides with phenol bearing either electron-withdrawing or electron-donating substituents was shown to give the corresponding products in excellent yields of 83-92% (Table 3,entries 1-5) with the exception of 4'-hydroxyacetophenone, which led to a low yield (17%) for the coupling reaction (Table 3,entry 9). This might be due to the decreased nucleophilicity of the phenolic hydroxyl group induced by the keto group. Slight electronic effects were observed when various phenols were submitted to coupling with para-methyl aryl iodides, because only slight decreases in the reaction yields were recorded (Table 3,entries 10-16 vs. 1,2,4-7). Whereas the O-arylation of various substituted phenols with 2-iodotoluene gave only moderate to good product yields (59-80%) (Table 3,entries 17- 21),which might be attributable to the influence of steric hindrance. As mentioned above,the results clearly indicated that steric hindrance had a huge influence on the coupling reaction. For instance,only 47% yield was obtained after 24 h under our standard reaction conditions when 2,6-dimethylphenol coupled with phenyl iodides (Table 3,entry 8). The results showed that the coupling of a or β-naphthol and aryl iodides (or 4-iodotoluene) proceeded in moderate to good yields (Table 3,entries 6,7,15 and 16). The results also revealed that an electron- withdrawing group in the aryl iodides favored the coupling reactions. For example,1- iodo-4-chlorobenzene successfully coupled with various phenols with higher yields than that for iodobenzene without an electronwithdrawing group (Table 3,entry 22,24-26 vs. 2-5). Similarly,the coupling reaction of benzyl alcohol with 1-iodo-4-chlorobenzene also smoothly afforded the expected ethers (3dk) in 90% yield (Table 3,entry 23). It was noteworthy that the coupling reaction of 2-acetylphenyl bromide with various substituted phenols gave good yields (73-90%) under our optimal conditions,although the aryl bromides are less reactive than the aryl iodides for the arylation reaction (Table 3,entries 27-31).
| Table 3 Cu-catalyzed coupling reaction of aryl iodides(bromides) with phenols (or benzyl alcohol).a |
In conclusion,2-((2-isopropylphenylimino)-methyl)phenol was found to be an efficient,inexpensive and facile ligand for the CuX-catalyzed O-arylation of phenols with aryl iodides and bromides. The present protocol is applicable to a variety of phenols and aryl iodides containing electron-withdrawing,electrondonating, and sterically demanding substrate combinations under mild conditions. Further study on the design and application of these new ligands in copper based Ullmann-type coupling reactions is currently ongoing. Acknowledgments
We are grateful to the National Natural Science Foundation of China and Key Laboratory of Organic Synthesis of Jiangsu Province for the financial support (Nos. 21201147,21302063,KJS1112). spx
| [1] | (a) J. Lindley, Tetrahedron report number 163: copper assisted nucleophilic substitution of aryl halogen, Tetrahedron 40 (1984) 1433-1456; (b) F. Theil, Synthesis of diaryl ethers: a long-standing problem has been solved, Angew. Chem. Int. Ed. Engl. 38 (1999) 2345-2347; (c) J.S. Sawyer, Recent advances in diaryl ether synthesis, Tetrahedron 56 (2000) 5045-5065; (d) S.V. Ley, A.W. Thomas, Modern synthetic methods for copper-mediated C(aryl)- O, C(aryl)-N, and C(aryl)-S bond formation, Angew. Chem. Int. Ed. Engl. 42 (2003) 5400-5449; (e) K. Kunz, U. Scholz, D. Ganzer, Renaissance of Ullmann and Goldberg reactionsprogress in copper catalyzed C-N-, C-O- and C-S-coupling, Synlett 15 (2003) 2428-2439; (f) F. Monnier, M. Taillefer, Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions: copper makes a difference, Angew. Chem. Int. Ed. Engl. 47 (2008) 3096- 3099. |
| [2] | (a) M.E. Jung, J.C. Rohloff, Organic chemistry of L-tyrosine. 1. General synthesis of chiral piperazines from amino acids, J. Org. Chem. 50 (1985) 4909-4913; (b) S.B. Singh, G.R. Pettit, Antineoplastic agents. 206. Structure of the cytostatic macrocyclic lactone combretastatin D-2, J. Org. Chem. 55 (1990) 2797-2800; (c) V.H. Deshpande, N.J. Gohkhale, Synthesis of combretastatin D-2, Tetrahedron Lett. 33 (1992) 4213-4216; (d) R. Nagarajan, D.A. Evans, K.M. DeViries, Glycopeptide Antibiotics (Drugs and the Pharmaceutical Sciences), Marcel Decker, New York, 1994, pp. 63-104; (e) S. Zenitani, S. Tashiro, K. Shindo, et al., A novel inhibitor of geranylgeranyl diphosphate synthase from Beauveria felina QN22047. I. Taxonomy, fermentation, isolation, and biological activities, J. Antibiot. 56 (2003) 617-621; (f) P. Cristau, J.P. Vors, J.P. Zhu, Rapid and diverse route to natural product-like biaryl ether containing macrocycles, Tetrahedron 59 (2003) 7859-7870; (g) C.W. Qian, Y. Pang, D. Fang, Q.S. Zong, Synthesis and bioactivities of new triazole compounds containing aryl ether, Chin. J. Pestic. Sci. 15 (2013) 256-260. |
| [3] | (a) G. Mann, J.F. Hartwig, Palladium alkoxides: potential intermediacy in catalytic amination, reductive elimination of ethers, and catalytic etheration. Comments on alcohol elimination from Ir(Ⅲ), J. Am. Chem. Soc. 118 (1996) 13109-13110; (b) M. Palucki, J.P. Wolfe, S.L. Buchwald, Synthesis of oxygen heterocycles via a palladium-catalyzed C-O bond-forming reaction, J. Am. Chem. Soc. 118 (1996) 10333-10334; (c) M. Palucki, J.P. Wolfe, S.L. Buchwald, Palladium-catalyzed intermolecular carbon-oxygen bond formation: a new synthesis of aryl ethers, J. Am. Chem. Soc. 119 (1997) 3395-3396; (d) A. Aranyos, D.W. Old, A. Kiyomori, et al., Novel electron-rich bulky phosphine ligands facilitate the palladium-catalyzed preparation of diaryl ethers, J. Am. Chem. Soc. 121 (1999) 4369-4378; (e) G. Mann, C. Incarvito, A.L. Rheigold, J.F. Hartwig, Palladium-catalyzed C-O coupling involving unactivated aryl halides. Sterically induced reductive elimination to form the C-O bond in diaryl ethers, J. Am. Chem. Soc. 121 (1999) 3224- 3225; (f) N. Kataoka, Q. Shelby, J.P. Stambuli, J.F. Hartwig, Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C-C, C-N, and C-O bondforming cross-couplings, J. Org. Chem. 67 (2002) 5553-5566; (g) D. Prim, J.M. Campagne, D. Joseph, B. Andrioletti, Palladium-catalysed reactions of aryl halides with soft, non-organometallic nucleophiles, Tetrahedron 58 (2002) 2041-2046; (h) A.V. Vorogushin, X.H. Huang, S.L. Buchwald, Use of tunable ligands allows for intermolecular Pd-catalyzed C-O bond formation, J. Am. Chem. Soc. 127 (2005) 8146-8149; (i) C.H. Burgos, T.E. Barder, X.H. Huang, S.L. Buchwald, Significantly improved method for the palladium-catalyzed coupling of phenols with aryl halides: understanding ligand effects, Angew. Chem. Int. Ed. Engl. 45 (2006) 4321-4326. |
| [4] | F. Ullmann, The Ullmann condensation and the synthesis of diarylamines, Ber. Dtsch. Chem Ges. 36 (1903) 2382-2384. |
| [5] | (a) J.F. Marcoux, S. Doye, S.L. Buchwald, A general copper-catalyzed synthesis of diarylethers, J. Am. Chem. Soc. 119 (1997) 10539-10540; (b) P.J. Fagan, E. Hauptman, R. Shapiro, A. Casalnuovo, Using intelligent/random library screening to design focused libraries for the optimization of homogeneous catalysts: Ullmann ether formation, J. Am. Chem. Soc. 122 (2000) 5043-5051; (c) C.W. Qian, S.J. Xu, Q.S. Zong, D. Fang, Copper-catalyzed synthesis of triarylamines from aryl halides and arylamines, Chin. J. Chem. 30 (2012) 1881-1885; (d) E. Buck, Z.J. Song, D. Tschaen, et al., Ullmann diaryl ether synthesis: rate acceleration by 2,2,6,6-tetramethylheptane-3,5-dione, Org. Lett. 4 (2002) 1623- 1626; (e) D. Ma, Q. Cai, H. Zhang, N,N-dimethyl glycine-promoted Ullmann coupling reaction of phenols and aryl halides, Org. Lett. 5 (2003) 3799-3802; (f) Q. Cai, B. Zou, D.W. Ma, Mild Ullmann-type biaryl ether formation reaction by Combination of ortho-substituent and ligand effects, Angew. Chem. Int. Ed. Engl. 45 (2006) 1276-1279; (g) Q. Cai, G. He, D.W. Ma, Mild and nonracemizing conditions for Ullmann-type diaryl ether formation between aryl iodides and tyrosine derivatives, J. Org. Chem. 71 (2006) 5268-5273; (h) A. Ouali, J.F. Spindler, H.J. Cristau, M. Taillefer, Mild conditions for coppercatalyzed coupling reaction of phenols and aryl iodides and bromides, Adv. Synth. Catal. 348 (2006) 499-505; (i) H.J. Cristau, P.P. Cellier, S. Hamada, J.F. Spindler, M. Tailefer, A general and mild Ullmann-type synthesis of diaryl ethers, Org. Lett. 6 (2004) 913-916; (j) H.H. Rao, Y. Jin, H. Fu, Y.Y. Jiang, Y.F. Zhao, A versatile and efficient ligand for copper-catalyzed formation of C-N, C-O, and P-C bonds: pyrrolidine-2-phosphonic acid phenyl monoester, Chem. Eur. J. 12 (2006) 3636-3646; (k) C. Palomo, M. Oiarbide, R. Lopez, E. Gomez-Bengoa, Phosphazene P4-But base for the Ullmann biaryl ether synthesis, Chem. Commun. (1998) 2091-2092; (l) X. Lü, W.L. Bao, A β-keto ester as a novel, efficient, and versatile ligand for copper(I)-catalyzed C-N, C-O, and C-S coupling reactions, J. Org. Chem. 72 (2007) 3863-3867; (m) A.B. Naidu, O.R. Raghunath, D.J.C. Prasad, G. Sekar, An efficient BINAM-copper( II) catalyzed Ullmann-type synthesis of diaryl ethers, Tetrahedron Lett. 49 (2008) 1057-1061; (n) Y. Chen, H. Chen, 1,1,1-Tris (hydroxymethyl) ethane as a new, efficient, and versatile tripod ligand for copper-catalyzed cross-coupling reactions of aryl iodides with amides, thiols, and phenols, Org. Lett. 8 (2006) 5609-5612; (o) T. Miao, L. Wang, Immobilization of copper in organic-inorganic hybrid materials: a highly efficient and reusable catalyst for the Ullmann diaryl etherification, Tetrahedron Lett. 48 (2007) 95-99; (p) D.A. Evans, J.L. Katz, T.R. West, Synthesis of diaryl ethers through the copperpromoted arylation of phenols with arylboronic acids. An expedient synthesis of thyroxine, Tetrahedron Lett. 39 (1998) 2937-2940; (q) Y. Zhao, Y. Wang, H. Sun, L. Li, H. Zhang, Ullmann reaction in tetraethyl orthosilicate: a novel synthesis of triarylamines and diaryl ethers, Chem. Commun. (2007) 3186-3188; (r) Q. Zhang, D.P. Wang, X.Y. Wang, K. Ding, (2-Pyridyl)acetone-promoted Cucatalyzed O-arylation of phenols with aryl iodides, bromides, and chlorides, J. Org. Chem. 74 (2009) 7187-7190; (s) J.W.W. Chang, S. Chee, S. Maka, et al., Copper-catalyzed Ullmann coupling under ligand- and additive-free conditions. Part 1: O-arylation of phenols with aryl halides, Tetrahedron Lett. 49 (2008) 2018-2022; (t) D. Maiti, S.L. Buchwald, Cu-catalyzed arylation of phenols: synthesis of sterically hindered and heteroaryl diaryl ethers, J. Org. Chem. 75 (2010) 1791-1794; (u) C.W. Qian, Q.S. Zong, D. Fang, Methenamine as an efficient ligand for coppercatalyzed coupling of phenols with aryl halides, Chin. J. Chem. 30 (2012) 199-203; (v) R.K. Gujadhur, C.G. Bates, D. Venkataraman, Formation of aryl-nitrogen, aryloxygen, and aryl-carbon bonds using well-defined copper(I)-based catalysts, Org. Lett. 3 (2001) 4315-4317; (w) R.K. Gujadhur, D. Venkataraman, Synthesis of |
| [6] | (a) K.C. Gupta, A.K. Sutar, Catalytic activities of Schiff base transition metal complexes, Coord. Chem. Rev. 252 (2008) 1420-1450; (b) R. Drozdzak, B. Allaert, N. Ledoux, et al., Ruthenium complexes bearing bidentate Schiff base ligands as efficient catalysts for organic and polymer syntheses, Coord. Chem. Rev. 249 (2005) 3055-3074; (c) L. Canali, D.C. Sherrington, Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis, Chem. Soc. Rev. 28 (1999) 85-93; (d) B.Y. Li, Y.R. Wang, Y.M. Yao, Y. Zhang, Q. Shen, Synthesis, structure and reactivity of samarium complexes supported by Schiff-base ligands, J. Organomet. Chem. 694 (2009) 2409-2414; (e) J. Cui, M.J. Zhang, Y.W. Zhang, Amino-salicylaldimine-palladium(II) complexes: new and efficient catalysts for Suzuki and Heck reactions, Inorg. Chem. Commun. 13 (2010) 81-85; (f) W. Yang, H. Liu, D.M. Du, Efficient in situ three-component formation of chiral oxazoline-Schiff base copper(II) complexes: towards combinatorial library of chiral catalysts for asymmetric Henry reaction, Org. Biomol. Chem. 8 (2010) 2956-2960; (g) W. Yang, D.M. Du, Highly enantioselective Henry reaction catalyzed by C2- symmetricmodular BINOL-oxazoline Schiff base copper(II) complexes generated in situ, Eur. J. Org. Chem. 2011 (2011) 1552-1556. |
| [7] | (a) F.S. Liu, Y.T. Huang, C. Lu, D.S. Shen, T. Cheng, Efficient salicylaldimine ligands for a palladium-catalyzed Suzuki-Miyaura cross-coupling reaction, Appl. Organomet. Chem. 26 (2012) 425-429; (b) N. Xie, Y. Chen, Design and synthesis of a selective chemosensor for Zn2+, Chin. J. Chem. 24 (2006) 1800-1803; (c) Q.H. Chen, J.L. Huang, Synthesis of novel zirconium complexes bearing mono- Cp and tridentate Schiff base [ONO] ligands and their catalytic activities for olefin polymerization, Appl. Organomet. Chem. 20 (2006) 758-765. |