b Department of Chemistry, Yasouj University, Yasouj 75918-74831, Iran;
c Department of Chemistry, Gachsaran Branch, Islamic Azad University, Gachsaran 75818-63876, Iran
Xanthenes and benzoxanthenes compounds have received great attention from many pharmaceutical and organic chemists because of their broad spectrum of biological and pharmaceutical properties,such as antibacterial [1],anti-inflammatory,and antiviral [2] properties. Moreover,these compounds are used as dyes [3],as fluorescent materials for visualization of biomolecules [4] and in laser technologies [5] because of their photochemical and photophysical properties. Various synthetic procedures have been developed for the preparation of xanthenediones. For example,copper iodide nanoparticles on poly(4-vinylpyridine) [6],trichloroisocyanuric acid and cyanuric chloride [7, 8],carbonbased solid acid [9],Fe3O4 and ZnO nanoparticles [10, 11], functionalized mesoporous materials [12],cellulose sulfuric acid and succinimide-N-sulfonic acid [13, 14],I2 [15],P2O5/Al2O3 [16] and DABCO-bromine [17] have been used as catalysts for the synthesis of xanthenediones. However,some of these methods involve the use of expensive reagents,toxic solvents,tedious workup,low yields,long reaction times and harsh reaction conditions. Therefore,there is a focus on developing an alternative, facile and green method for the synthesis of xanthenes derivatives.
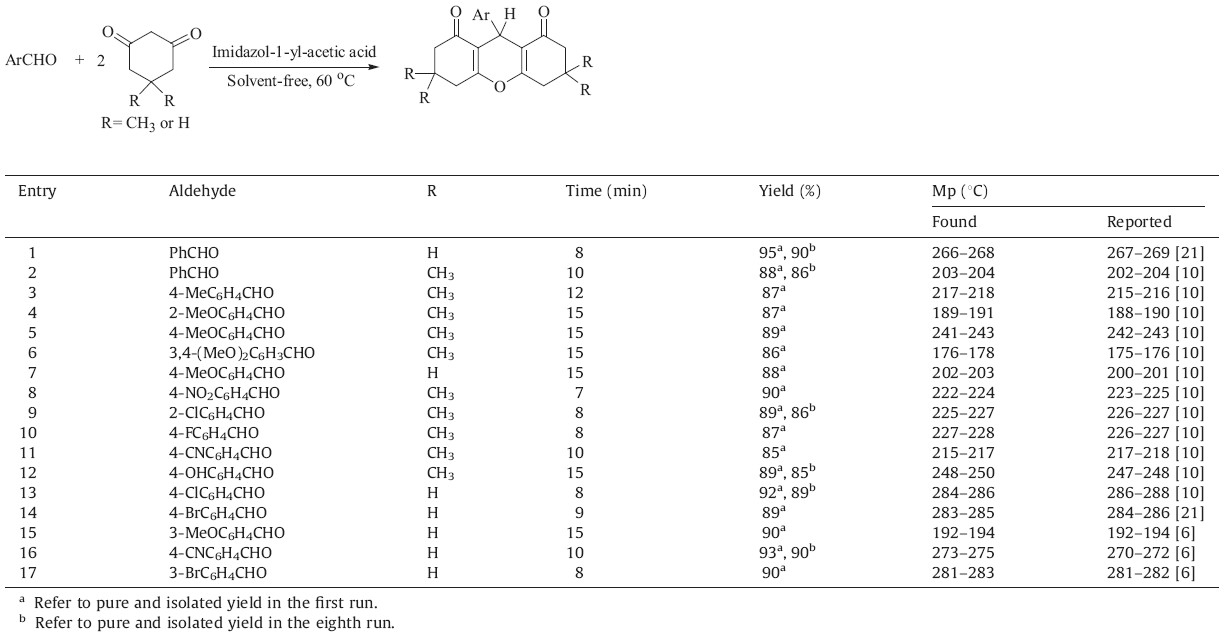
Organocatalytic reactions are becoming dominant tools for the production of complex molecular skeletons [18]. Organocatalysts are organic molecules that do not contain any metal which accelerate reactions in substoichimetric amounts. Bifunctional organocatalysts are organocatalysts with dual action. Imidazol-1- yl-acetic acid is an example of a bifunctional organocatalysts. This amino acid has two forms: a neutral and an ionized form. It is almost always in the ionized form (a in Scheme 1),except in the gaseous phase and in aprotic solvents (b in Scheme 1). Herein,we report imidazol-1-yl-acetic acid as a new,efficient and recyclable green bifunctional organocatalyst for the synthesis of 1,8- dioxooctahydroxanthenes under solvent-free conditions.

|
Download:
|
| Scheme 1.The two forms of imidazol-1-yl-acetic acid. | |
All of the products were prepared by our own procedures; their spectroscopic and physical data were compared with those of authentic samples [6, 10, 21]. NMR spectra were recorded in CDCl3 on Bruker AC 400 MHz instrument spectrometers using TMS as the internal standard. IR spectra were recorded on a BOMEMMB-Series 1998 FT-IR spectrometer. Elemental analyses were performed on a Thermo Finnigan CHNS-O analyzer,1112 series. Chemicals were purchased from Fluka,Merck and Aldrich Chemical companies.Yields refer to isolated pure products.
To a mixture of 5,5-dimethyl-1,3-cyclohexanedione or 1,3- cyclohexanedione (4 mmol) and aldehyde (2 mmol),imidazol-1- yl-acetic acid (1.2 mmol) was added and the reaction mixture was stirred at 60℃ for 7-15 min (Table 1). The progress of the reaction was monitored by TLC (eluent:EtOAc/n-hexane = 5/1,v/v). After completion of the reaction,the mixture was cooled to room temperature,water (20 mL) was added and the mixture was stirred for 10 min. The mixture was filtered and the solid residue was recrystallized from ethanol to afford the pure product. Evaporation of the filtrate gave the recovered catalyst which purified by recrystallization in cool methanol (30 mL),dried at 50℃ and used in the next consecutive runs.
9-(3-Methoxyphenyl)-3,3,6,6-tetramethyl-2,3,4,5,6,7,8,9-octahydro- 1H-1,8-xanthenedion (Table 1,entry 15): White solid,mp 192-194℃ [6]; IR (KBr,cm-1): νmax 3070,2950,1650,1605,1580, 1450,1360,1265,1220,1200,1180,1130,1050,960; 1H NMR (400 MHz,CDCl3): δ 1.99-2.1 (m,4H),2.3-2.45 (m,4H),2.54-2.71 (m,4H),3.81 (s,3 H),4.83 (s,1H),6.69-6.71 (dd,1H,J1 = 8.0 Hz, J2 = 1.6 Hz),6.89-6.95 (m,2H),7.17 (t,1H,J = 8.0 Hz); 13C NMR (100 MHz,CDCl3): δ 20.3,27.2,31.5,37.0,55.2,111.5,114.5,116.8, 121.0,129.0,146.0,159.4,164.0,196.5. Elem. Anal. Calcd. for C20H20O4: C,74.06%; H,6.21%; Found: C,74.05%; H,6.22%.
9-(4-Cyanophenyl)-3,3,6,6-tetramethyl-2,3,4,5,6,7,8,9-octahydro- 1H-1,8-xanthenedion (Table 1,entry 16):White solid,mp 270- 272℃ [6]; IR(KBr,cm-1): νmax 3070,2950,2900,2220,1652,1619, 1356,1200,1173,1125,958,830,610,550; 1H NMR (400 MHz, CDCl3): δ 1.95-2.11 (m,4H),2.30-2.42 (m,4H),2.57-2.73 (m,4H),4.84 (s,1H),7.44 (d,2H,J = 8.0 Hz,),7.53 (d,2H,J = 8.0 Hz); 13C NMR (100 MHz,CDCl3): δ 20.2,27.1,32.3,36.8,110.2,115.8,119.10, 129.4,132.0,149.7,164.5,196.5. Elem. Anal. Calcd. for C20H20O4: C, 74.06%; H,6.21%; Found: C,74.05%; H,6.22%.
9-(3-Bromophenyl)-3,3,6,6-tetramethyl-2,3,4,5,6,7,8,9-octahydro- 1H-1,8-xanthenedion (Table 1,entry 17): White solid,mp 281-282℃ [6]; IR (KBr,cm-1): νmax 3070,2910,2890,1660,1620, 1560,1470,1420,1358,1200,1170,1122,957,800,680; 1H NMR (400 MHz,CDCl3): δ 1.95-2.1 (m,4H),2.30-2.44 (m,4H),2.55-2.63 (m,2H),2.66-2.73 (m,2H),4.79 (s,1H),7.11 (t,1H,J = 7.6 Hz), 7.26-7.36 (m,3H); 13C NMR (100 MHz,CDCl3): δ 20.3,27.2,31.6, 36.9,116.3,122.3,127.7,129.6,131.0,146.6,164.3,196.4. Elem. Anal. Calcd. for C20H17NO4: C,75.22%; H,5.37%; N,4.39; Found: C, 75.22%; H,5.39%; N,4.39%. 3. Results and discussion
Imidazol-1-yl-acetic acid contains both acidic and basic functionalities. The existence of such functionalities makes this molecule an ideal bifunctional organocatalyst for condensation reactions [19]. In fact the bifunctional catalytic activities of imidazol-1-yl-acetic acid arise from its ionized form (a in Scheme 1). To the best of our knowledge there is only one report that has investigated the catalytic activity of this simple and interesting bifunctional organocatalyst [20]. Consequently,we decided to study the potential catalytic activity of this simple bifunctional catalyst for the preparation of 1,8-dioxooctahydroxanthenes under solvent-free conditions.
In an initial attempt,a mixture of 1,3-cyclohexadione (4 mmol) and benzaldehyde (2 mmol) in the presence of imidazol-1-ylacetic acid (0.6 mmol,75 mg) was stirred at room temperature. After 30 min,48% of the expected product 3 (Scheme 2) was obtained. Another run was performed using 1.2 mmol (150 mg) of imidazol-1-yl-acetic acid which led to 88% of the title compound after 30 min. To improve the yield and rate of the reaction,the same reaction was carried out under solvent-free conditions using 1.2 mmol of imidazol-1-yl-acetic acid at 60℃. A significant improvement in the rate of the reaction was observed and the reaction time was decreased to 8 min. Moreover,the yield of the title compound was increased to 95%. Greater amounts of the catalyst did not clearly improve the reaction rate. When the same reaction was performed in the absence of the catalyst,only trace amount of corresponding product was obtained even after a long reaction time (1 h).
|
Download:
|
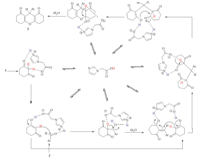
| Scheme 2.The proposed mechanism for the synthesis of xanthene derivatives using imidazol-1-yl-acetic acid as bifunctional organocatalyst. | |
To extend the scope of the reaction and to generalize the procedure,a variety of electronically divergent aromatic aldehydes, 5,5-dimethyl-1,3-cyclohexanedione or 1,3-cyclohexanedione,were examined under optimized reaction conditions and the results are summarized in Table 1.
| Table 1 Imidazol-1-yl-acetic acid catalyzed synthesis of 1,8-dioxooctahydroxanthenes under solvent-free conditions. |
In all cases,aromatic aldehydes carrying either an electronwithdrawing group or an electron-donating group in the ortho, meta and para positions reacted effectively and gave the products in good to excellent yields. It is observed that substituents in the aromatic ring of aldehydes have a mild effect on the reaction times. Aromatic aldehydes with electron-withdrawing groups reacted faster than those with electron-donating groups.
A simple and clean procedure was used for the separation of the catalyst from the reaction mixture. After completion of the reaction (Table 1),the mixture was cooled and water was added then stirred for 10 min. The undissolved solid was filtered and washed with water. Recrystallization from hot ethanol afforded pure 1,8-dioxooctahydroxanthenes. In order to recover the catalyst,the filtrate was evaporated to dryness and recrystallized from cool methanol. The obtained precipitate was filtered and dried at 50℃. The recovered catalyst was reused in the next consecutive similar runs (7 runs). No appreciable yield decrease was observed during reusing processes. Typically,the yield difference between the first and 8th runs of the reaction between benzaldehyde and 1,3-cyclohexadione (Table 1,entry 1) was only 5%. These observations indicate that the efficiency and catalytic activity of the title catalyst is almost completely maintained over 8 runs. In all cases,the corresponding 1,8-dioxooctahydroxanthene was isolated in typically good to high yields (85-93%). The catalytic activity of imidazol-1-yl-acetic acid most probably arises from its dual action participating in acid catalyzed as well as base catalyzed activation during the reaction progress. The suggested mechanism is illustrated in Scheme 2.
During the reaction progress,the two forms of imidazol-1-ylacetic acid (a and b in Scheme 1) are in equilibrium with each other. Form a of the catalyst plays two roles,works both as acid and base. These activities allow it to be a bifunctional organocatalyst for the preparation of 1,8-dioxooctahydroxanthenes. The advantages of the introduced catalyst are its cheapness,green nature and ease of workup and catalyst recovery. More importantly,its bifunctional activity can efficiently catalyze this typical condensation reaction by simultaneous activation of both acidic and basic sites during the condensation progress (Scheme 2). 4. Conclusion
Imidazole-1-yl-acetic acid was used as a green bifunctional organocatalyst for the preparation of 1,8-dioxooctahydroxanthene derivatives under solvent free conditions. Aromatic aldehydes were efficiently reacted and gave the desired products in high yields. The easily recoverable catalyst was used in 8 consecutive runs and no appreciable yield decrease was observed during reusing processes. Acknowledgments
We are thankful to the Islamic Azad University,Sousangerd Branch and Yasouj University for the financial support of this work.
| [1] | A.M. El-Brashy, M. El-Sayed Metwally, F.A. El-Sepai, Spectrophotometric determination of some fluoroquinolone antibacterials by binary complex formation with xanthene dyes, Farmaco 59 (2004) 809-817. |
| [2] | K. Chibale, M. Visser, D.V. Schalkwyk, et al., Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents, Tetrahedron 59 (2003) 2289-2296. |
| [3] | B.B. Bhowmik, P. Ganguly, Photophysics of xanthene dyes in surfactant solution, Spectrochim. Acta 61 (2005) 1997-2003. |
| [4] | C.G. Knight, T. Stephens, Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles, Biochem. J. 258 (1989) 683-689. |
| [5] | M. Ahmad, T.A. King, D.K. Ko, B.H. Cha, J. Lee, Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases, J. Phys. D: Appl. Phys. 35 (2002) 1473-1476. |
| [6] | J. Albadi, M. Keshavarz, M. Abedini, M. Khoshakhlagh, Copper iodide nanoparticles on poly(4-vinylpyridine): a new and efficient catalyst for the synthesis of 1,8- dioxooctahydroxanthenes under solvent-free conditions, J. Chem. Sci. 125 (2013) 295-298. |
| [7] | M.A. Bigdeli, F. Nemati, G.H. Mahdavinia, H. Doostmohammadi, A series of 1,8- dioxooctahydroxanthenes are prepared using trichloroisocyanuric acid, Chin. Chem. Lett. 20 (2009) 1275-1278. |
| [8] | Z. Zhang, P. Zhang, S.H. Yang, H.J. Wang, J. Deng, Multicomponent, solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one derivatives catalyzed by cyanuric chloride, J. Chem. Sci. 122 (2010) 427-432. |
| [9] | V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, M. Mahdavi, Highly efficient synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes catalysed by carbon-based solid acid under solvent-free conditions, Synth. Commun. 39 (2009) 4328-4340. |
| [10] | B. Karami, S.J. Hoseini, K. Eskandari, A. Ghasemi, H. Nasrabadi, Synthesis of xanthene derivatives by employing Fe3O4 nanoparticles as an effective and magnetically recoverable catalyst in water, Catal. Sci. Technol. 2 (2012) 331-338. |
| [11] | J. Safaei-Ghomi, M.A. Ghasemzadeh, Zinc oxide nanoparticles: a highly efficient and readily recyclable catalyst for the synthesis of xanthenes, Chin. Chem. Lett. 23 (2012) 1225-1229. |
| [12] | J. Mondal, M. Nandi, A. Modak, A. Bhaumik, Functionalized mesoporous materials as efficient organocatalysts for the syntheses of xanthenes, J. Mol. Catal. A: Chem. 363-364 (2012) 254-264. |
| [13] | J. Venu-Madhav, Y. Thirupathi-Reddy, P. Narsimha-Reddy, et al., Cellulose sulfuric acid: an efficient biodegradable and recyclable solid acid catalyst for the one-pot synthesis of aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions, J. Mol. Catal. A: Chem. 304 (2009) 85-87. |
| [14] | F. Shirini, N. Ghaffari-Khaligh, Succinimide-N-sulfonic acid: an efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions, Dyes Pigments 95 (2012) 789-794. |
| [15] | R.Z. Wang, L.F. Zhang, Z.S. Cui, Iodine-catalyzed synthesis of 12-aryl-8,9,10,12- tetrahydro-benzo[a]xanthen-11-one derivatives via multicomponent reaction, Synth. Commun. 39 (2009) 2101-2107. |
| [16] | A. Zarei, A.R. Hajipour, L. Khazdooz, The one-pot synthesis of 14-aryl or alkyl-14Hdibenzo[a,j]xanthenes catalyzed by P2O5/Al2O3 under microwave irradiation, Dyes Pigments 85 (2010) 133-138. |
| [17] | M. Bigdeli, Clean synthesis of 1,8-dioxooctahydroxanthenes promoted by DABCObromine in aqueous media, Chin. Chem. Lett. 21 (2010) 1180-1182. |
| [18] | (a) Special issue about organic catalysis, Adv. Synth. Catal. 346 (9-10) (2004) 1007-1249; (b) Hot Topics, Wiley-VCH, Wurttemberg, 2011, This is a selection of recent articles in field of organocatalyst from Angew. Chem, Chem. Eur. J, Eur. J. Org. Chem and Adv. Synth. Catal.. |
| [19] | T. Okino, Y. Hoashi, Y. Takemoto, Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts, J. Am. Chem. Soc. 125 (2003) 12672-12673. |
| [20] | M. Kargar, R. Hekmatshoar, A. Mostashari, Z. Hashemi, Efficient and green synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones using imidazol-1-ylacetic acid as a novel, reusable and water-soluble organocatalyst, Catal. Commun. 15 (2011) 123-126. |
| [21] | D. Fang, K. Gong, Z.L. Liu, Synthesis of 1,8-dioxo-octahydroxanthenes catalyzed by acidic ionic liquids in aqueous media, Catal. Lett. 127 (2009) 291-295. |