Functionalization of carbon materials,in particular,singlewalled carbon nanotubes (SWCNTs) has attracted considerable attention and interest of researchers because of their numerous applications [1, 2, 3, 4]. Recently,the azide-modified graphitic [5], nanofiber [6] and other carbon nanomaterials [7] have shown advantages because the azide groups could react with alkyne derivatives specifically through the Cu(I)-catalyzed Huisgen 1,3- dipolarcycloaddition (the ‘‘click’’ chemistry). So it provides a useful platform for the attachment of a wide variety of species onto the carbon’s surface. At the same time,the azide-modified gold [8] and silicon [9] have also been reported. However,the current method for introducing azido groups on carbon materials by reacting with IN3 [5, 6, 7] has a shortcoming: iodide substituted products were also present.
Electrochemical method represents a useful route for covalent functionalization of carbon surface [10]. The electrochemical reduction of aryl diazonium salts [11] and oxidation of primary amines [12] have provided a variety of functionalized carbon materials. However,to our knowledge,there were no reports on azide-functionalization of carbon or other materials by the electrochemical method to date. Herein,we describe an effective azidation process for carbon nanotubes by electrochemical oxidation of sodium azide. And its application on other carbon materials was also discussed. 2. Experimental 2.1. Preparation of the SWCNTs film (SWCNT-P)
SWCNTs were purchased from XF NANO Inc. made by the CVD method. The range of diameter is 1-2 nm and the length is 5- 30 μm. 50 mg of SWCNTs was sonicated in 50 mL of 1% Triton X- 100 aqueous solution for 2-3 h. The dispersion was filtrated with a 0.45 μm-pore PTFE membrane filter (Ø = 50 μm) under vacuum, washed by a large amount of water and methanol in alternate. Then the filter was dried for 24 h at room temperature. The resulting film was peeled off from the PTFE membrane and annealed at 500℃ under argon flow for 4 h. 2.2. Electrochemical functionalization
The cyclic voltammetric response for the electrochemical oxidation of 1 mol/L of NaN3 on SWCNT film is shown in Fig. S1 (Supporting information). It depicts the appropriate functionalization potential for the oxidation of N3- is 0.7 V vs. SCE. In a threeelectrode cell,with a SCE reference electrode and a Pt counter electrode,the prepared SWCNTs film was used as a working electrode and immersed in a NaN3 solution (1 mol/L) at room temperature. A potential of 0.7 V vs. SCE was applied on the SWCNTs electrode for 30 min. After soaked in H2O for 4 h to remove the unreacted NaN3,the film was rinsed with a large quantity of methanol. The azide-functionalized nanotubes (SWCNT-N3) were obtained after drying under vacuum at room temperature for 24 h. 3. Results and discussion 3.1. Infrared spectroscopy
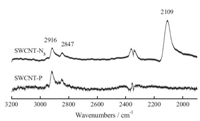
The surface groups of two SWCNTs were characterized using single internal reflection infrared spectroscopy (ATR-FTIR) and are shown in Fig. 1. Compared with the as-prepared SWCNT-P,the most significant peak in the spectrum of SWCNT-N3 was the asymmetric stretch of the organic azide group,which occurs at 2109 cm-1 [13]. And,the intensity of the carbon-hydrogen stretches absorption peaks at 2916 and 2847 cm-1 decreased in SWCNT-N3. Furthermore,the νs(N-H) band of HN3 at around 3100 cm-1 is absent [14] in both spectra. All the above-mentioned evidence supported the assignment of the formation of a covalent C-N bond that superseded by the former C-H group.
|
Download:
|
| Fig. 1.ATR-FTIR spectra of SWCNT-P and SWCNT-N3 | |
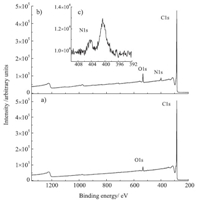
The groups on SWCNTs surfaces were also characterized by Xray photoelectron spectroscopy (XPS) and the spectra were shown in Fig. 2. It could be seen that only C1s and O1s peaks (Fig. 2a) present in the spectrum of SWCNT-P,while an additional N1s peak (Fig. 2b) could be observed in the spectrum of SWCNT-N3. Furthermore,an N1s narrow scan shows the N1s peak could be distinguished to two distinct peaks (Fig. 2c) with a peak area ratio of 2:1. Since sodium signal was not observed from the SWCNT-N3 sample surface (Table S1,Supporting information),it could be concluded that the N1s arise from an covalent linked N3 group and the ~404 eV peak could assigned to the central,electron-deficient N,and the ~400 eV peak to the N bonded to nanotubes and the terminal N. The similar XPS peaks were observed on the N3-surface [8] and N3-/Si(1 1 1) surface [15].
|
Download:
|
| Fig. 2.XPS spectra of (a) SWCNT-P,(b) SWCNT-N3 and (c) N1s narrow scan of SWCNT-N3. | |
The results of surface element analysis on SWCNTs are shown in the Table S1. For SWCNT-N3,the amount of the N element is 3.0% and the C element is 91.9%. The surface coverage of nitrogen can be calculated as one N3 group per 92 carbon atoms approximately. 3.3. TGA/MS analysis
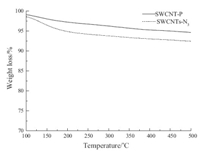
Further evidence for the presence of covalent azide group on SWCNT-N3 is provided by thermogavimetric analysis (TGA) of the SWCNTs (Fig. 3). Obvious difference in weight loss between SWCNT-N3 and SWCNT-P samples has been observed. The former lost much more weight during the measurement. The MS analysis of the evolution products from two SWCNT is shown in Figs. S2 and S3 (Supporting information). And the ion current for the radical fragment of N2 (m/z = 28,14) of SWCNT-N3 is more concentrated than that of SWCNT-P. In addition,the fragment of CN [m/z = 26] and CNH [m/z = 27] could only be detected from SWCNT-N3. This evidence could lead to the conclusion thatN3 groups present on the surface of SWCNT-N3 through a covalent interaction. Similar conclusion was obtained from the amides-functionalization of carbon nanotubes [16].
|
Download:
|
| Fig. 3.Weight loss plot for SWCNT-N3 and SWCNT-P. | |
The degree of functionalization of SWCNT-N3 could be estimated from the loss of weight with a value of about 2%,which can be translated into one N3 group per approximately 170 carbon atoms of the SWCNT framework. It is understandable that the value is less than the XPS result,which is calculated according to the N surface element analysis (3.0%). 4. Conclusion
Azide-functionalized single-walled carbon nanotubes (SWCNTN3) were obtained by the electrochemical oxidation of a SWCNT containing membrane in a N3- aqueous solution. The presence of covalently attached azide groups on the surface of SWCNT was confirmed by a variety of spectroscopic tools,including ATR-FTIR,XPS and TGA/MS. So this will open the door to a broad variety of azide-functionalized materials,especially carbon materials like graphitic carbon,carbon fibers and glassy carbon. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.11.023.
| [1] | D. Tasis, N. Tagmatarchis, A. Bianco, M. Prato, Chemistry of carbon nanotubes, Chem. Rev. 106 (2006) 1105-1136. |
| [2] | N. Karousis, N. Tagmatarchis, Current progress on the chemical modification of carbon nanotubes, Chem. Rev. 110 (2010) 5366-5397. |
| [3] | M.A. Khalilzadeh, H.K. Maleh, A. Amiri, F. Gholami, R.M. Mazhabi, Determination of captopril in patient human urine using ferrocenemonocarboxylic acid modified carbon nanotubes paste electrode, Chin. Chem. Lett. 21 (2010) 1467-1470. |
| [4] | M. Ahmadipour, M.A. Taher, H. Beitollahi, R. Hosseinzadeh, Electrocatalytic determination of L-cysteine using a modified carbon nanotube paste electrode: application to the analysis of some real samples, Chin. Chem. Lett. 23 (2012) 981- 984. |
| [5] | A. Devadoss, C.E.D. Chidsey, Azide-modified graphitic surface for covalent attachment of alkyne-terminated molecules by ""click"" chemistry, J. Am. Chem. Soc. 129 (2007) 5370-5371. |
| [6] | E.C. Landis, R.J. Hamers, Covalent grafting of redox-active molecules to vertically aligned carbon nanofiber arrays via ""click"" chemistry, Chem. Mater. 21 (2009) 724-730. |
| [7] | A. Devadoss, N.K. Devaraj, C.E.D. Chidsey, Preparation of azide-modified carbon surfaces for coupling to various species related applications, US Patent C07C247/14, 20110184196, 2011-2-28. |
| [8] | J.P. Collman, N.K. Devaraj, T.P.A. Eberspacher, C.E.D. Chidsey, Mixed azide-terminated monolayers: a platform for modifying electrode surfaces, Langmuir 22 (2006) 2457-2464. |
| [9] | S. Ciampi, T. Bocking, K.A. Kilian, et al., Functionalization of acetylene-terminated monolayers on Si(1 0 0) surfaces: a click chemistry approach, Langmuir 23 (2007) 9320-9329. |
| [10] | C.G. Liu, H.T. Fang, F. Li, M. Liu, H.M. Cheng, Single-walled carbon nanotubes modified by electrochemical treatment for application in electrochemical capacitors, J. Power Sources 160 (2006) 758-760. |
| [11] | J.L. Bahr, J.P. Yang, D.V. Kosynkin, et al., Functionalization of carbon nanotubes by electrochemical reduction of ary diazonium salts: a bucky paper electrode, J. Am. Chem. Soc. 123 (2001) 6536-6542. |
| [12] | S.E. Kooi, U. Schlicht, M. Burghard, K. Kern, Electrochemical modification of single carbon nanotubes, Angew. Chem. Int. Ed. 41 (2002) 1353-1355. |
| [13] | E. Lieber, C.N.R. Ran, T.S. Chan, C.W.W. Hoffman, Infrared spectra of organic azides, Anal. Chem. 29 (1957) 916-918. |
| [14] | S.R. Carlo, J. Torres, D.H. Fairbrother, Thermal and electron-induced reactions of hydrazoic acid (HN3) adsorbed on gold and ice, J. Phys. Chem. B 105 (2001) 6148- 6157. |
| [15] | P.G. Cao, K. Xu, J.R. Heath, Azidation of silicon(1 1 1) surfaces, J. Am. Chem. Soc. 130 (2008) 14910-14911. |
| [16] | Z. Syrgiannis, F. Hauke, J. Röhrl, et al., Covalent sidewall functionalization of SWNTs by nucleophilic addition of lithium amides, Eur. J. Org. Chem. (2008) 2544-2550. |




