b National Center for Nanosicence and Technology, Beijing 100190, China;
c China Banknote Ink Company, Shanghai 201315, China
Luminescent substances have been intensively applied in the field of bio-detection in fluorescence immunoassays [1, 2, 3, 4, 5],DNA detection [6, 7, 8, 9, 10, 11] and bio-imaging [12, 13, 14, 15, 16, 17, 18, 19, 20]. Luminescent lanthanide complexes are promising for bio-detection because of their long luminescence lifetimes,large Stokes shifts,and narrow-line emission [21, 22, 23, 24]. More importantly,when luminescent lanthanide complexes are used as bio-probes,the interference of shortlived background fluorescence from the biological tissue and light scattering from the instrument on the luminescence imaging is so little that an enhanced signal-to-noise ratio is obtained. Unfortunately, most luminescent lanthanide complexes are excited by ultraviolet (UV) light [25, 26],which damages biological samples and has a short penetration depth. To overcome these probλems,it is necessary to develop luminescent lanthanide complexes that respond to longer wavelengths of light,such as visible light [27,28,29,30,31,32,33,34]. Furthermore,to realize the application of luminescent lanthanide complexes in bio-detection,bio-probes based on luminescent lanthanide complexes with long excitation wavelength need to be developed.
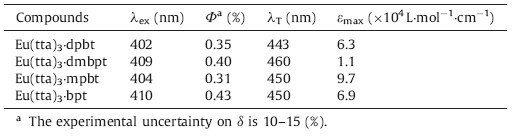
A few europium complexes excited by visible light have been reported [27, 28, 29, 30, 31, 32, 33, 34],typical examples including Eu(tta)3·dpbt [27] (tta = thenoyltrifluoroacetonate; dpbt = 2-(N,N-diethylanilin-4- yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine) (Scheme 1a),Eu(tta)3·dmbpt [29] (dmbpt = 2-(N,N-diethyl-2,6-dimethylanilin- 4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine) (Scheme 1b),Eu(tta)3·bpt(bpt = 2-(N,N-di-ethylanilin-4-yl)-4,6- bis(pyrazol-1-yl)-1,3,5-triazine) (Scheme 1c),and Eu(tta)3·mpbt [30] (mpbt = 2-(N,N-diethylanilin-4-yl)-4,6-bis(3-methylpyrazol- 1-yl)-1,3,5-triazine) (Scheme 1d). Their photophysical data is summarized in Table 1. Eu(tta)3·bpt,with the longest wavelength and highest quantum efficiency,became the best choice for luminescence imaging among these europium complexes. Moreover, the way of energy transfer for Eu(tta)3·bpt being probably dominated by a singlet energy-transfer pathway breaks the restriction of excitated-triplet-state energy level and contributes to the excellent visible-light-sensitized luminescence efficiency. However,because of its bad solubility in water and instability in polar solvents,such as DMF,THF and alcohols,it is difficult to directly apply Eu(tta)3·bpt as a bio-probe in bioassay applications. Therefore,to meet the demand of applying Eu(tta)3·bpt as a bioprobe, treatment of Eu(tta)3·bpt to solve these probλems must be pursued.
|
Download:
|
| Scheme 1.Molecularstructures of Eu(tta)3·dpbt (a),Eu(tta)3·dmbpt (b),Eu(tta)3·bpt(c) and Eu(tta)3·mpbt (d). | |
| Table 1 Photophysical data for Eu(tta)3·dpbt,Eu(tta)3·dmbpt,Eu(tta)3·bpt and Eu(tta)3·mbpt in toluene. Excitation maximal wavelengths (λex),extinction coefficients (εmax),fluorescence quantum yields (Φ),excitation maximal window(λT). |
One viable way to prepare colloidal nanoparticles based on the compλex Eu(tta)3·dpbt has been tried. The two-photon-excitation imaging of Eu(tta)3·dpbt nanoparticles in live cancer cells was reported by Wang et al. [35, 36, 37]. The nanoparticles were prepared by encapsulating Eu(tta)3·dpbt in hydrophobic cores of waterdispersible biocompatible nanoparticles of poly(methyl methacrylate- co-methacrylic acid),showing good luminescence properties, dispersion stability,and photostability in water solution. However,the visible-light-sensitized luminescence properties of the compλex Eu(tta)3·dpbt in nanoparticles were still to be improved. Another way is preparing europium nanoparticles with the ligand dpbt,as presented by Yuan et al. [38, 39, 40, 41]. Their preparation processes are mainly composed of two steps: first,the tetradentate β-diketonate-Eu3+-dpbt complexes were covalently bound to silane derivatives to form functionalized precursors; second,the nanoparticles were obtained by the copolymerization of the above conjugates and silane reagents in a water-in-oil reverse microemulsion. However,some probλems appeared in these nanoparticles,such as strong scattering to visible light (λ > 450 nm) and the weak absorption peak of europium compλex in the UV-visible absorption spectrum.
Keeping the above probλems in mind,in this work we prepared a new kind of hybrid nanoparticle based on the Eu(tta)3·bpt compλex (EuHNPS) by a co-precipitation-condensation method [42, 43],in which 1H,1H,2H,2H-perfluorooctyltrimethoxysilane (PFOTS) and poly(styrene-co-methyl methacrylate) [P(ST-co- MMA)] were used as matrix materials. The results demonstrated the nanoparticles displayed excellent long-wavelength-sensitized luminescent properties. Their maximal excitation wavelength was located at 425 nm,and their luminescence quantum yield was measured to be 0.22 (room temperature). The photostability of the prepared nanoparticles was also measured to check its future application in bio-detection. 2. Experimental
Materials and methods: 1H,1H,2H,2H-perfluorooctyltrimethoxysilane (PFOTS) (PFOTS,97%) was purchased from Matrix Scientific. Poly(styrene-co-methyl methacrylate) (P(ST-co-MMA), 40% styrene,Mw ~ 100,000-150,000) was purchased from Sigma- Aldrich. Hexadecyltrimethyl ammonium bromide (CTAB) (≥99%) was obtained from Acros Organics. Other chemicals of AR grade were used as received. Eλemental analysis of EuHNPS was carried out with an Eλementarvario EL eλemental analyzer. Eu and Si contents of EuHNPS were determined by an inductively coupled plasma atomic emission spectrometer (ICP). Transmission electron microscopy (TEM) was taken on a transmission electron microscope (JEM 2000FX,Hitachi). Energy dispersive X-ray spectroscopy (EDX) measurements,scanning transmission electron microscope (STEM),and eλemental mapping were carried out on a field emission transmission microscope (Tecnai G2F20 U-TWIN) with an energy dispersive X-ray spectroscope (EDX,EPMA-1600). UV- vis absorption and photoluminescence measurements were carried out on an absorption spectrometer (UV-2550,SHIMADZU) and a fluorescence spectrophotometer (RF-5301PC,SHIMADZU). The photoluminescence decay kinetics of EuHNPS colloidal nanoparticles were measured by FLS920 (Edinburgh Instruments). Luminescence quantum yield (Φ) of the prepared nanoparticles was determined according to the method described by Demas and Grosby [44],using DCM (4-dicyanomethylene-2-methyl-6-pdimethylaminostyryl- 4H-pyran) in n-propanol (Φ = 0.57 ± 0.02) as the reference. The photo-bleaching experiments were carried out on a fluorescence spectrophotometer using a 150Wxenon lamp as an excitation source. Eu(tta)3·bpt was synthesized according to the method reported previously [30].
Preparation of EuHNPS: A colloidal solution of EuHNPS was synthesized by a co-precipitation-condensation method [42, 43]. In a typical experiment,2.0 mL of acetone solution containing PFOTS (1.14 × 10-3 mol L-1),P(ST-co-MMA) (0.12 g L-1),and Eu(tta)3·bpt (1.20 × 10-4 mol L-1) was dropwise added into an aqueous solution of CTAB (7.0 mL,1.40 × 10-3 mol L-1) with stirring at room temperature. The mixture was then stirred for another 30 min to obtain a yellow colloidal solution. Subsequently, the as-prepared colloidal solution was centrifuged at 10,000 × g to remove large particles. The supernatant was then centrifuged at 35,000 × g,and the obtained precipitation was redispersed in 8 mL of water to prepare a colloidal solution of EuHNPS. This process was repeated to remove most of the CTAB and produce a stable colloid solution of EuHNPS (about 50.2 mg L-1,corresponding to a yield of EuHNPS of 26 wt%) with an average diameter of 60 nm as measured by TEM. A colloidal solution of nanoparticles was also prepared by the aforementioned processes except for the addition of P (ST-co-MMA) and PFOTS,respectively.
PFOTS and Silicon content determination of EuHNPS: the 25 mL EuHNPS solution was centrifuged at 35,000 × g to collect the resulting EuHNPS precipitate,which was then placed in the desiccator equipped with P2O5 and dried for 20 h. The dried EuHNPS solids were added into 1 mL of acetone,dispersed for 50 min by ultrasound,and allowed to stand for 24 h. Finally,the EuHNPS were fully swollen and released the unhydrolyzed PFOTS. 0.2 mL of the above suspension and 0.2 mL of methanol were mixed to precipitate P (ST-co-MMA) and centrifuged at 10,000 × g. The resulting supernatant was analyzed by gas chromatography to determine the content of the unhydrolyzed PFOTS. The remaining 0.8 mL of acetone suspension was digested with 5 mL of concentrated nitric acid (guaranteed reagent) and perchloric acid (guaranteed reagent) to obtain a clear solution at atmospheric pressure,diluted to constant volume,and the silicon content digestion solution by ICP. 3. Results and discussion
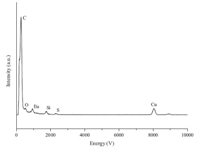
The ideal bio-probes are not only water-soluble but stable at physiological pH and the in presence of biological fluids. To prepare the bio-probe based on Europium compλex,the Eu(tta)3·bpt was encapsulated in a water-soluble nanoparticle by adding the mixture of PFOTS,P(ST-co-MMA),and Eu(tta)3·bpt into an aqueous solution of CTAB. The obtained nanoparticles (EuHNPS) can be well dispersed in pure water or Tris-HCl buffer solution (10 mmol L-1, pH 7.8). On the other hand,no precipitation can be found in the prepared EuHNPS hydrosol even after several months,which indicates its promising application in bio-detection. The TEM images of EuHNPS show that the nanoparticle size ranged from 10 to 70 nm Fig. 1(a)-(c)]. The STEM (scanning transmission electron microscope) image [Fig. 1(d)] of the EuHNPS revealed that the nanoparticle had a core/shell (Eu/Si) structure,and eλement mapping of S (Fig. 1e) and Eu (Fig. 1f) in the selected area further confirmed the structure of EuHNPS. The energy dispersive X-ray spectroscope (EDX) indicated that the EuHNPS includes C,Si,Eu, and S eλements,as shown in Fig. 2.

|
Download:
|
| Fig. 1.TEM images (a),(b),(c) of EuHNPS and STEM of EuHNPS (d),eλement mapping of S (e) and Eu (f). | |
|
Download:
|
| Fig. 2.The energy dispersive X-ray spectroscope of EuHNPS. | |
In order to determine the hydrolysis proportion of PFOTS in EuHNPS nanoparticles,the unhydrolyzed PFOTS in the EuPHS nanoparticles was washed with acetone,and the quantity of PFOTS was measured by the gas chromatography. The rest Si content in the washed EuPHS nanoparticles was measured by ICP. The results demonstrated that the proportion of PFOTS formed into the polysiloxane was 85%. The hydrolysis of surface PFOTS led to the formation of the polysiloxane shell due to the presence of water molecules on the surface of EuHNPS nanoparticles.
P (ST-co-MMA) rapidly precipitated in water,while the hydrolysis of PFOTS in a neutral aqueous solution was a relatively slow process. As a result,we believe the formation of EuHNPS probably happened in two steps. Firstly,after the acetone solution of P (ST-co-MMA),PFOTS,and Eu(tta)3·bpt was added into the aqueous solution of CTAB,nanoparticles of the hydrophobic polymer P(ST-co-MMA) containing hydrophobic Eu(tta)3·bpt and PFOTS quickly formed by precipitation. Then, hydrolytic condensation of PFOTS occurred on the surfaces of the prepared nanoparticles; subsequently,the polysiloxane shells formed.
Fig. 3 shows the UV-vis absorption spectra of Eu(tta)3·bpt molecules in the colloidal nanoparticles and in toluene solution. Compared with the maximal absorption peak and peak shape (~350 nm) of the ligand tta in Eu(tta)3·bpt toluene,those of the ligand tta in Eu(tta)3·bpt colloidal solution did not change significantly. Importantly,the maximal absorption wavelength of bpt in colloidal solution (425 nm) red-shifted 15 nmcompared to that of bpt in toluene (410 nm) in Fig. 3(a). The absorption intensity in the long wavelength domain for the ideal fluorescence bionanoprobe should be relatively strong. Recently,Yuan et al. reported the preparation of long wavelength-excited europiumdoped silica nanoparticles using a synthesized visible lightexcited tetradentade-diketonate-Eu3+-bpt ternary compλex (BHHD-Eu3+-bpt) (BHHD: 2,6-bis(10,10,10,20,20,30,30-heptafluoro- 40,60-hexanedion-60-yl)dibenzothiophene) as a luminoprobe [41]. Unfortunately,the absorption peak of europium compλex in the nanoparticles was much smaller in the visible light region. However,in our work,the fluorescence excitation window of Eu(tta)3·bpt in colloidal solution extends up to 480 nm,which shifts about 30 nmtoward the long wavelength direction relative to that of Eu(tta)3·bpt in toluene in Fig. 3(b).

|
Download:
|
| Fig. 3.Spectra of Eu(tta)3·bpt molecules in the colloidal nanoparticles (-) and in toluene solution (- -). (a) UV-vis absorption spectra; (b) fluorescence excitation spectra(λem = 620 nm); (c) decay curve of luminescence at 620 nm of the colloidal nanoparticles (λex = 425 nm),the blue line is the fit curve by the second order exponential decay;(d) photoluminescence spectra (λex = 435 nm). The Eu(tta)3·bpt concentration in all of these samples is 4.0×10-6 mol L-1. | |
The formation of J-aggregates around the polar compound Eu(tta)3·bpt probably contributes to the bathochromic-shift of the absorption peak [36, 37]. The formation of J-aggregates usually brings about red-shift of the absorption peak and fluorescence excitation peak,which often occurs for organic dyes. However,the above phenomenon is limited for lanthanide complexes. For example,the excitation peak of the nanoparticles based on the photosensitized ligand bpt and the auxiliary ligand BHHD did notexhibit obvious bathochromic-shift [41].
The photoluminescence decay kinetics upon excitation wavelength of 620 nm could be accounted by a two-exponential decay model function,which yielded the decay time constants of 0.47 ms (65%) and 0.17 ms (35%) [Fig. 3(c)]. Being different from the case of EuHNPS,the luminescence decay profile of Eu(tta)3·bpt molecules in toluene was single-exponential decay,and the luminescence lifetime was measured to be 0.40 ms,which indicated that more than one kind of stacking mode existed for the Eu(tta)3·bpt molecules in EuHNPS.
The luminescence quantum yield and molar extinction coefficient of Eu(tta)3·bpt nanoparticles protected by polymer were measured to be 0.22 and 2.8 × 104 L·mol-1·cm-1 (λex: 425 nm),respectively. As a result,the luminescence brightness (ε Φ) of EuHNPS was calculated to be 6.2 × 103 L·mol-1·cm-1, which was approximately half that of Eu(tta)3·bpt compλex in toluene (room temperature). In addition,the value (ε Φ) was 4.3 × 103 L·mol-1·cm-1 upon excitation at 435 nm,whichwas 2.3 times as great as that of Eu(tta)3·bpt compλex in toluene [Fig. 3(d)], indicatingmore prominent long-wavelength-sensitized luminescence of these nanoparticles.
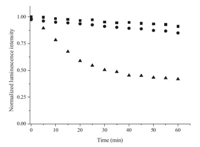
Assembling the Eu(tta)3·bpt into nanoparticles not only extended the excitation window but had a positive effect on the photostability,a key factor for luminescent bionanoprobes. The photostability of the prepared EuHNPS was measured by its photo-bleaching behaviors. Meanwhile,the luminescence decay experiments of Eu(tta)3·bpt nanoparticles protected by P(ST-co-MMA) (EuPSM) and polysiloxane (EuPSO) as a contrast were performed. As shown in Fig. 4,after continuous irradiation with a 150W Xenon lamp for 1 h,the luminescence intensity of EuHNPS,EuPSM,and EuPSO decreased by 10%,15%,and 58%, respectively. The experimental results demonstrated that the matrix materials had an important effect on the luminescence intensity for Eu(tta)3·bpt nanoparticles. After irradiation for 1 h,the luminescence intensity of EuPSO showed the largest decline among EuHNPS,EuPSM and EuPSO,indicating that hydroxyl groups generated by the hydrolysis of PFOTS probably quenched the fluorescence. Under the same illumination condition,the luminescence intensity of EuPSM reduced by 15%,indicating that polymer P(ST-co-MMA) had a positive effect on enhancing the luminescence stability of europium nanoparticles. In addition,EuHNPS displayed much better photostability than EuPSM and EuPSO due to the formation of a silica shell structure,which protected Eu(tta)3·bpt molecules from being attacked by solvent and other molecules.
|
Download:
|
| Fig. 4.SPhotostability curve of the Eu(tta)3·bpt nanoparticles.■: EuHNPS; •: EuPSM;▲: EuPSO; (λex = 425 nm; λem= 620 nm). | |
In summary,a new kind of hybrid nanoparticle (EuHNPS) based on the luminescent compλex Eu(tta)3·bpt that is excited by visible light has been prepared with a co-precipitation-condensation encapsulation method. TEM analysis results revealed that the EuHNPS has a core/shell structure. EuHNPS exhibited good stability,dispersibility,and high photostability in aqueous solutions. The spectra results of EuHNPs had the strongest excitation wavelength of Eu3+ luminescence located at 425 nm with a red-edge up to 480 nm,and the quantum yield of Eu3+ luminescence was measured to be 0.22 upon light-excitation at 425 nm. Moreover,the photostability of EuHNPS was excellent. Considering the excellent visible-light-excited luminescence properties of EuHNPS,we believe that it is a promising luminescent material for the development of bionanoprobes. Acknowledgment
This work was financially supported by the scientific research foundation of Shandong Province Outstanding Young Scientist Award (No. BS2011SW031).
| [1] | T. Soukka, H. Härmä, J. Paukkunen, T. Lövgren, Utilization of kinetically enhanced monovalent binding affinity by immunoassays based on multivalent nanoparticle- antibody bioconjugates, Anal. Chem. 73 (2001) 2254-2260. |
| [2] | M.Q. Tan, G.L. Wang, X.D. Hai, Z.Q. Ye, J.L. Yuan, Development of functionalized fluorescent europium nanoparticles for biolabeling and time-resolved fluorometric applications, J. Mater. Chem. 14 (2004) 2896-2901. |
| [3] | J.L. Yuan, K. Matsumoto, H. Kimura, A new tetradentate β-diketonate-europium chelate that can be covalently bound to proteins for time-resolved fluoroimmunoassay, Anal. Chem. 70 (1998) 596-601. |
| [4] | I. Hemmilä, V. Laitala, Progress in lanthanides as luminescent probes, J. Fluoresc. 15 (2005) 529-542. |
| [5] | J.L. Yuan, G.L. Wang, Lanthanide-based luminescence probes and time-resolved luminescence bioassays, Trends Anal. Chem. 25 (2006) 490-500. |
| [6] | Y. Chen, Y.M. Chi, H.M. Wen, Z.H. Lu, Sensitized luminescent terbium nanoparticles: preparation and time-resolved fluorescence assay for DNA, Anal. Chem. 79 (2007) 960-965. |
| [7] | Y. Chen, Z.H. Lu, Dye sensitized luminescent europium nanoparticles and its timeresolved fluorometric assay for DNA, Anal. Chim. Acta 587 (2007) 180-186. |
| [8] | K. Hashino, K. Ikawa, M. Ito, et al., Application of a fluorescent lanthanide chelate label on a solid support device for detecting DNA variation with ligation-based assay, Anal. Biochem. 364 (2007) 89-91. |
| [9] | A. Son, A. Dhirapong, D.K. Dosev, et al., Rapid and quantitative DNA analysis of genetic mutations for polycystic kidney disease (PKD) using magnetic/luminescent nanoparticles, Anal. Bioanal. Chem. 390 (2008) 1829-1835. |
| [10] | A.M. Nonat, S.J. Quinn, T. Gunnlaugsson, Mixed f-d coordination complexes as dual visible- and near-infrared-emitting probes for targeting DNA, Inorg. Chem. 48 (2009) 4646-4648. |
| [11] | D.A. Heller, E.S. Jeng, T.K. Yeung, et al., Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes, Science 311 (2006) 508-511. |
| [12] | A. Bodi, K.E. Borbas, J.I. Bruce, Near IR-emitting DNA-probes exploiting stepwise energy transfer processes, Dalton Trans. 38 (2007) 4352-4358. |
| [13] | A. D'Alé o, G. Pompidor, B. Elena, et al., Two-photon microscopy and spectroscopy of lanthanide bioprobes, ChemPhysChem 8 (2007) 2125-2132. |
| [14] | A. Picot, A. D'Alé o, P.L. Baldeck, et al., Long-lived two-photon excited luminescence of water-soluble europium compλex: applications in biological imaging using two-photon scanning microscopy, J. Am. Chem. Soc. 130 (2008) 1532-1533. |
| [15] | G.L. Law, K.L. Wong, C.W.Y. Man, et al., Emissive terbium probe for multiphoton in vitro cell imaging, J. Am. Chem. Soc. 130 (2008) 3714-3715. |
| [16] | G.L. Law, K.L.Wong, C.W.Y.Man, S.W. Tsao,W.T.Wong, A two-photon europium compλex as specific endoplasmic reticulum probe, J. Biophoton. 2 (2009) 718-724. |
| [17] | B. Song, G.L. Wang, M.Q. Tan, J.L.A. Yuan, Europium(Ⅲ) compλex as an efficient singlet oxygen luminescence probe, J. Am. Chem. Soc. 128 (2006) 13442-13450. |
| [18] | J.C.G. Bünzli, A.S. Chauvin, C.D.B. Vandevyver, B. Song, S. Comby, Lanthanide bimetallic helicates for in vitro imaging and sensing, Ann. N. Y. Acad. Sci. 1130 (2008) 97-105. |
| [19] | B.Y. Wu, H.F. Wang, J.T. Chen, X.P. Yan, Fluorescence resonance energy transfer inhibition assay for a-fetoprotein excreted during cancer cell growth using functionalized persistent luminescence nanoparticles, J. Am. Chem. Soc. 133 (2011) 686-688. |
| [20] | S.W. Yang, H. Li, Assaying dynamic cell-cell junctional communication using noninvasive and quantitative fluorescence imaging techniques: LAMP and infrared- LAMP, Nat. Protoc. 4 (2009) 94-101. |
| [21] | J.C.G. Bünzli, A.S. Chauvin, H.K. Kim, E. Deiters, S.V. Eliseeva, Lanthanide luminescence efficiency in eight- and nine-coordinate complexes: role of the radiative lifetime, Coord. Chem. Rev. 254 (2010) 2623-2633. |
| [22] | J.C.G. Bünzli, S. Comby, A.S. Chauvin, C.D.B. Vandevyver, New opportunities for lanthanide luminescence, J. Rare Earths 25 (2007) 257-274. |
| [23] | J.L. Zhang, B.W. Chen, X. Luo, K. Du, Eu(Ⅲ) compλex-doped PMMA having fast radiation rate and high emission quantum efficiency, Chin. Chem. Lett. 23 (2012) 945-948. |
| [24] | H.Q. Chen, J. Xu, F. Yuan, et al., A ""turn off"" luminescence resonance energy transfer aptamer sensor based on near-infrared upconverting NaYF4:Yb3+, Tm3+ nanoparticles as donors and gold nanorods as acceptors, Chin. Chem. Lett. 24 (2013) 79-81. |
| [25] | J.C.G. Bünzli, C. Piguet, Taking advantage of luminescent lanthanide ions, Chem. Soc. Rev. 34 (2005) 1048-1077. |
| [26] | G.S. He, L.S. Tan, Q. Zheng, P.N. Prasad, Multi-photon absorbing materials: molecular designs, syntheses, characterizations, and applications, Chem. Rev. 108 (2008) 1245-1330. |
| [27] | C. Yang, L.M. Fu, Y. Wang, et al., A highly luminescent europium compλex showing visible-light-sensitized red emission: direct observation of the singlet pathway, Angew. Chem. Int. Ed. 43 (2004) 5010-5013. |
| [28] | L.M. Fu, X.F. Wen, X.C. Ai, et al., Efficient two-photon-sensitized luminescence of a europium(Ⅲ) compλex, Angew. Chem. Int. Ed. 44 (2005) 747-750. |
| [29] | R. Hao, M.Y. Li, Y. Wang, et al., A europium compλex with excellent two-photonsensitized luminescence properties, Adv. Funct. Mater. 17 (2007) 3663-3669. |
| [30] | F.M. Xue, Y. Ma, L.M. Fu, et al., A europium compλex with enhanced longwavelength sensitized luminescent properties, Phys. Chem. Chem. Phys. 12 (2010) 3195-3202. |
| [31] | J.C.G. Bünzli, Lanthanide luminescence for biomedical analyses and imaging, Chem. Rev. 110 (2010) 2722-2729. |
| [32] | V. Divya, V. Sankar, K.G. Raghu, et al., A mitochondria-specific visible-light sensitized europium β-diketonate compλex with red emission, Dalton Trans. 42 (2013) 12317-12323. |
| [33] | V. Divya, M.L.P. Reddy, Visible-light excited red emitting luminescent nanocomposites derived from Eu3+-phenathrene-based fluorinated β-diketonate complexes and multi-walled carbon nanotubes, J. Mater. Chem. C 1 (2013) 160-170. |
| [34] | J. Xu, Z.H. Sun, L. Jia, et al., Visible light sensitized attapulgite-based lanthanide composites: microstructure, photophysical behaviour and biological application, Dalton Trans. 40 (2011) 12909-12916. |
| [35] | G.S. Shao, R.C. Han, Y. Ma, et al., Bionanoprobes with excellent two-photonsensitized Eu3+ luminescence properties for live cell imaging, Chem. Eur. J. 16 (2010) 8647-8651. |
| [36] | G.S. Shao, F.M. Xue, R.C. Han, M.X. Tang, Y. Wang, Synthesis and characterization of europium compλex nanoparticles with long-wavelength sensitized luminescence, Acta Phys. Chim. Sin. 26 (2010) 2031-2036. |
| [37] | X.Y. Fu, G.S. Shao, R.C. Han, et al., Nanoprobes with enhanced two-photonsensitized Eu3+ luminescence properties for live cell imaging, Acta Phys. Chim. Sin. 28 (2012) 2480-2486. |
| [38] | J. Wu, G.L. Wang, D.Y. Jin, et al., Luminescent europium nanoparticles with a wide excitation range from UV to visible light for biolabeling and time-gated luminescence bioimaging, Chem. Commun. 3 (2008) 365-367. |
| [39] | J. Wu, Z.Q. Ye, G.L. Wang, et al., Visible-light-sensitized highly luminescent europium nanoparticles: preparation and application for time-gated luminescence bioimaging, J. Mater. Chem. 19 (2009) 1258-1264. |
| [40] | L.N. Jiang, J. Wu, G.L. Wang, et al., Development of a visible-light-sensitized europium compλex for time-resolved fluorometric application, Anal. Chem. 82 (2010) 2529-2535. |
| [41] | L. Tian, Z.C. Dai, L. Zhang, et al., Preparation and time-gated luminescence bioimaging applications of long wavelength-excited silica-encapsulated europium nanoparticles, Nanoscale 4 (2012) 3551-3557. |
| [42] | Y. Wang, X.Y. Fu, G.S. Shao, Photoluminescent nanoparticle, preparation, and application, China, CN 200910203407.3 [P] 2010. |
| [43] | H.S. Peng, M.I.J. Stich, J.B. Yu, et al., Luminescent europium(Ⅲ) nanoparticles for sensing and imaging of temperature in the physiological range, Adv. Mater. 22 (2010) 716-719. |
| [44] | J.N. Demas, G.A. Crosby, The measurement of photoluminescence quantum yields, J. Phys. Chem. 75 (1971) 991-1024. |