b College of Chemistry and Chemical Engineering, Luoyang Normal University, Luoyang 471022, China
The rational design and construction of coordination polymers (CPs) has recently attracted considerable interest,owing to their potential application as functional materials in molecular magnetism, catalysis,fluorescent sensing,and gas sorption [1, 2, 3, 4].Although CPs catalysis is a relatively new field,more and more related works are being reported by many researchers [5]. Compared with conventional homogeneous catalysts,the use of CPs as heterogeneous catalysts has many advantages,including separation and recovery,disposal of spent catalysts,and so on [6, 7].
So far,a lot of CPs,which are constructed out of cadmium (II) and organic bridging units,including a variety of organocarboxylate ligands,have been extensively documented [8]. However,in comparison with other organic acid anions such as carbonates and phosphonates,the coordination chemistry of organosulfonates is relatively rare,probably for their weak coordinating ability, despite similar structures [9]. Indeed,the efforts of many groups have recently evidenced that the use of different amines alters the size and nature of the organic region. In the right chemical environment of metal ions,the SO3- group can compete with water molecules and coordinate to metal ions [10, 11, 12].
Among the examples reported,arenedisulfonates have been found to be some of the best candidates for the construction of intriguing CPs with novel architectures because they have rigid spacers and multiple potential binding sites,showing various coordination modes ranging from μ1 to μ6 [12, 13]. Meanwhile,the introduction of a second ligand possessing multifunctionality may alter the structures and properties of these complexes [9, 10, 11, 12, 13]. The tripodal ligand 2,4,5-tri(4-pyridyl)-imidazole (tpim) is an efficient and versatile organic building unit for construction of coordination architectures and has been widely used in the previous studies [14]. Inspired by these,in this paper,we employed 1,5-naphthalenedisulfonates as bridging ligands and introduced 2,4,5-tri(4-pyridyl)- imidazole as a co-ligand into the organosulfonate system. By this strategy,we successfully synthesized one new Cd(II) arenedisulfonate coordination polymer,[Cd(tpim)(1,5-nds)]n (1). Furthermore, for its catalysis toward epoxide ring-opening reactions,the luminescent and thermal properties have been determined. 2. Experimental
2.1. Materials and physical techniques
All reagents were commercially available and used as received. The bridging ligand tpim was synthesized according to the literature method [15]. Elemental analyses (C,H,N) were performed by a PerkinElmer 240 elemental analyzer. IR spectrum was recorded as KBr pellets on a Nicolet-6700 spectrometer in the 4000-400 cm-1 range. TG measurement was performed by heating the crystalline sample from 25℃ to 850℃ at a rate of 10℃/min in a N2 atmosphere on a SDTQ600 differential thermal analyzer. Powder X-ray diffraction (PXRD) measurements were performed on a Bruker D8-ADVANCE X-ray diffractometer with Cu Ka radiation (λ= 1.5418Å ). The crystal structure determination was performed on a Bruker smart 1000 CCD diffractometer equipped with graphite-monochromatized Mo Kα radiation (λ= 0.71073Å ). 1H NMR spectra were recorded on a Varian UNITY/NOVA 400 NMR spectra meter using CDCl3 as the solvent at room temperature. Chemical shifts are given in δ relative to TMS. The coupling constants J are given in Hz.
The synthesis and structural characterizations of polymer 1 are described in the Supporting information. The crystallographic data (Table S1) and other details on the refinement,bonds,lengths,and angles (Table S2) are also provided in the Supporting information. Crystallographic data for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication No.CCDC-947842. 2.2. Typical procedure for the ring-opening reactions of epoxides
The ring-opening reactions of the epoxides were carried out at room temperature. In a typical aminolysis reaction,styrene oxide (0.5 mmol) was treated with aniline (0.5 mmol) in the presence of catalyst 1 (0.01 mmol). The reactions were monitored by thin layer chromatography. After 4 h,the catalyst was filtered off,and the filtrate was concentrated under reduced pressure. The crude product was purified by flash column chromatography on silica gel using a hexane/ethyl acetate mixture (10:1) as the eluent. The products were isolated and analyzed by NMR techniques. The recovered catalyst was washed with ethyl acetate,dried,and reused without further purification or regeneration. Moreover,the recovered catalysts were characterized by X-ray powder diffraction and showed identical results to those of the fresh samples. 3. Results and discussion
3.1. Description of the structures of 1
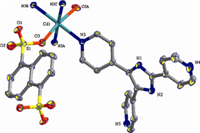
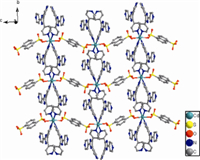
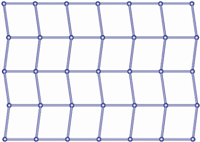
The X-ray crystallographic analysis reveals that 1 is a 2D coordination network. In the asymmetrical unit,there are half Cd(II) ion,half 1,5-nds ligand,and one tpim co-ligand. As illustrated in Fig. 1,each Cd(II) atom adopts a distorted octahedral coordination sphere consisting of four pyridyl nitrogen atoms from four individual tpim ligands and two oxygen atoms from two individual sulfonates. The N3,N3A,N5B,and N5C comprise the equatorial plane,and O3 and O3A are located in the axial positions. The Cd-N bond lengths are 2.333(2) and 2.366(2) Å , respectively,and the Cd-O distance is 2.3335(18)Å . They are comparable to previously reported values [14]. As depicted in Fig. 2,the 1,5-nds ligands bridge the Cd(II) atoms to form a 1D polymeric chain. Each [Cd(1,5-nds)]n polymeric chain is further linked by tpim ligands to construct a 2D sheet with 4(4).6(2) topology (Fig. 3). The Cd...Cd distances separated by the sulfonate groups of 1,5-nds and a pair of tpim are 12.188 (2) and 10.712 (4)Å,respectively.
|
Download:
|
| Fig. 1.Coordination environment of Cd(II) in complex 1 with the ellipsoids drawn at the 30% probability level. Symmetry code: (A) -x + 1/2,y,-z + 1/2; (B) -x + 1/2,y - 1,-z + 1/2; (C) x,y - 1,z. | |
|
Download:
|
| Fig. 2.View of 2D sheet in 1. | |
|
Download:
|
| Fig. 3.Schematic description of the 4-connected 2D network in1. | |
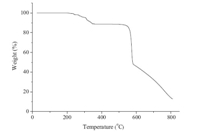
The thermogravimetric analysis (TGA) revealed that 1 was stable up to 210℃ (Fig. 4). The TG curve of 1 exhibits three weight loss stages. The first of 10.9% at 210-355℃ corresponds to partial decomposition of organic ligands. The second and third weight losses at 518-810℃ can be attributed to the decomposition of the framework,forming CDO as a final product (observed 12.98%, calcd. 13.0%). The pure polymer 1 was confirmed by powder X-ray diffraction (PXRD) measurements in which diffraction peaks of experimental data were in excellent agreement with the simulated data from single-crystal X-ray data (Fig. S1 in Supporting information).
|
Download:
|
| Fig. 4.Thermogravimetric analysis (TGA) curve for 1. | |
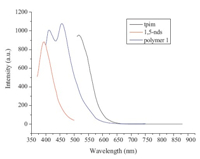
Emission behavior of polymer 1,1,5-nds,and tpim was studied in solid state at room temperature (Fig. 5). Tpim and 1,5-nds employed to synthesize polymer 1 exhibit good fluorescent emission at 518 nm (λex = 494 nm) and 394 nm (λex = 345 nm), respectively. Upon excitation at 380 nm,1 displayed exhibits two emission maxima at 411 and 456 nm. The observed blue-shifted (62 nm) of polymer 1 may be assigned to an intraligand emission state as reported for Cd(II) or other d10 metal complexes with Ndonor ligands [16]. The fluorescent intensity for 1 is much stronger compared to the free ligands,possibly resulted from the enhancement of rigidity of the ligand after the coordination effect and the reduction of the loss of energy by radiation less decay [14].
|
Download:
|
| Fig. 5.Emission spectra of polymer 1 and its constituting ligands. | |
CPs catalysts have recently gained prominence as a catalyst for epoxide ring-opening reactions because these reactions provide important synthetic tools for generating stereo-controlled fine chemicals [17]. In the previous reports,two Cu-MOFs were successful as heterogeneous catalysts for amino alcohol synthesis [18]. In this paper,the CP 1 was examined as a heterogeneous catalyst for the aminolysis reaction of epoxides under solvent-free conditions. The results are summarized in Table 1. It is noted that the CP 1 showed good catalytic properties (Table 1,entries 1-7). The product yields are not very sensitive to reaction substrates in the experiment.
| Table 1 The ring-opening reactions of epoxides with amines catalyzed by 1 a. |
Several control experiments were performed to confirm that the CP 1 was indeed the catalytic source for epoxide ring-opening. In initial control reactions without CP 1,the reaction did not proceed at all,whereas the use of Cd(CH3COO)2·2H2O resulted in very little conversion. Likewise,after carrying out the complete heterogeneous reaction of epoxides aminolysis,the catalyst was filtered off,and then fresh substrate and solvent were added to the recovered catalyst: the reactions showed significant loss in activity after several consecutive experiments (for example,~2% drop in the third and fourth run,Table 1,entry 1). After each of them, filtrate was used in a new reaction and no catalytic activity was found. These control experiments strongly suggest that the reaction was promoted by heterogeneous catalysis by CP 1. Meanwhile, the recovered CPs remained stable with complete retention of reactivity,which is further confirmed by PXRD analysis. As shown in Fig. S2 (in Supporting information),the recovered material shows no change in the structure of the catalyst. 4. Conclusion
In summary,we have identified one new 2D cadmium naphthalenedisulfonate complex. With the help of pyridine groups,the SO3- groups can act as potential monodentate ligands and compete with water molecules for Cd(II) coordination,similar to that of previous reports [9, 10, 11, 12, 13]. Further research demonstrates that the resultant networks display high catalytic activity in the epoxide ring-opening reaction of epoxides and amines. Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No.21272109),the Natural Science Foundation of Henan Province (No.112300410138),and the Foundation of the Education Department of Henan Province (No.2011B150021),and Luoyang Science and Technology Fund. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2013.11.045. y
| [1] | H.L. Jiang, Q. Xu, Porous metal-organic frameworks as platforms for functional applications, Chem. Commun. 47 (2011) 3351-3370. |
| [2] | T. Rahi, M. Baghernejad, K. Niknam, Synmesis of a-aminonitriles using silicabonded N-propylpiperazine sulfamic acid as a recyclable catalyst, Chin. Chem. Lett. 23 (2012) 1103-1106. |
| [3] | H.H. Wu, Z.M. Zhang, E.B. Wang, Synthesis and structure of a pure inorganic polyoxo-metalate-based porous framework, Chin. Chem. Lett. 23 (2012) 355-358. |
| [4] | A. Corma, H. García, F.X. LIabré s i Xamena, Engineering metal organic frameworks for heterogeneous catalysis, Chem. Rev. 110 (2010) 4606-4655. |
| [5] | J.Y. Lee, O.K. Farha, J. Roberts, et al., Metal-organic framework materials as catalysts, Chem. Soc. Rev. 38 (2009) 1450-1459. |
| [6] | L.Q. Ma, C. Abney, W.B. Lin, Enantioselective catalysis with homochiral metalorganic frameworks, Chem. Soc. Rev. 38 (2009) 1248-1256. |
| [7] | J.A. Zhao, S.F. Chen, D.D. Zhao, et al., The structure and luminescence properties of three complexes based on bifunctional imidazole-dicarboxylate connector, Chin. Chem. Lett. 24 (2013) 483-486. |
| [8] | D.B. Shi, Y.W. Ren, H.F. Jiang, J.X. Lu, X.F. Cheng, A new three-dimensional metalorganic framework constructed from 9, 10-anthracene dibenzoate and Cd(Ⅱ) as a highly active heterogeneous catalyst for oxidation of alkylbenzenes, Dalton Trans. 42 (2013) 484-491. |
| [9] | J.W. Cai, Structural chemistry and properties of metal arenesulfonates, Coord. Chem. Rev. 248 (2004) 1061-1083. |
| [10] | Z.X. Lian, J.W. Cai, C.H. Chen, H.B. Luo, Linear silver isonicotinamide complex extended by arenedisulfonate via hydrogen bonds and weak Ag. . .O interactions, CrystEngComm 9 (2007) 319-327. |
| [11] | T.L. Hu, W.P. Du, B.W. Hu, et al., Novel Ag(I) complexes with azole heterocycle ligands bearing acetic acid group: synthesis, characterization and crystal structures, CrystEngComm-10 (2008) 1037-1043. |
| [12] | J.P. Zhao, B.W. Hu, F.C. Liu, et al., Arenedisulfonate-lanthanide supramolecular architectures with phenanthroline as a co-ligand: syntheses and structures, CrystEngComm 9 (2007) 902-906. |
| [13] | C.H. Chen, J.W. Cai, C.Z. Liao, et al., Variation in the coordination mode of arenedisulfonates: syntheses and structural characterization of mononuclear and dinuclear cadmium(Ⅱ) arenedisulfonate complexes with two- to zero-dimensional architectures, Inorg. Chem. 41 (2002) 4967-4974. |
| [14] | X.Q. Liang, X.H. Zhou, C. Chen, et al., Self-assembly of metal-organic coordination polymers constructed from a versatile multipyridyl ligand: diversity of coordination modes and structures, Cryst. Growth Des. 9 (2009) 1041-1053. |
| [15] | M.V. Proskumina, N.A. Lozinskaya, S.E. Tkachenko, N.S. Zefirov, Reaction of aromatic aldehydes with ammonium acetate, Russ. J. Org. Chem. 38 (2002) 1149-1153. |
| [16] | X. Li, B.L. Wu, C.Y. Niu, Y.Y. Niu, H.Y. Zhang, Syntheses of metal-2-(pyridin-4-yl)- 1H-imidazole-4,5-dicarboxylate networks with topological diversity: gas adsorption, thermal stability and fluorescent emission properties, Cryst. Growth Des. 9 (2009) 3423-3431. |
| [17] | L.E. Martinez, J.L. Leighton, D.H. Carsten, E.N. Jacobsen, Highly enantioselective ring opening of epoxides catalyzed by (salen)Cr(Ⅲ) complexes, J. Am. Chem. Soc. 117 (1995) 5897-5898. |
| [18] | K. Tanaka, S. Oda, M. Shiro, A novel chiral porous metal-organic framework: asymmetric ring opening reaction of epoxide with amine in the chiral open space, Chem. Commun. 7 (2008) 820-822. |





