b Department of Chemistry, Faculty of Science, University of Kurdistan, Sanandaj 720, Iran
Development of simple,safe,ecofriendly,andeconomical synthetic routes for diverse organic compounds from readily available reagents are one of the major challenges and a fascinating task in organic synthesis. In this field,one-step methods are more convenient in comparison to the multistep ones,since they often require shorter reaction time and give higher yields with easier workup procedures. Nowadays,one-pot multicomponent condensation reactions are popular for the synthesis of heterocyclic compounds [1]. Multicomponent reactions (MCRs) constitute a special attractive synthetic strategy,since they provide easy and rapid access to large libraries of organic compounds with diverse substitution patterns. So one-pot multicomponent reactions are easier to carry out than multistep syntheses [2].
Polyfunctionalized heterocyclic compounds play important roles in the drug discovery processes,and an analysis of drugs in late development or on the market,shows that 68% of them are heterocycles [3]. As an important heterocyclic derivative,the pyrrolo[2,1-c][1, 4]benzoxazines are novel tricyclic compounds, some of which have biological activity [4,5,6]. However,the broad potential biological activities for these compounds have not yet been fully explored perhaps due to the lack of general methods for the synthesis of the unique tricyclic core. The reported methods forsynthesizing such compounds often involve multiple reactionsteps with low efficiency and difficulties in introducing different functional groups [7,8,9,10].
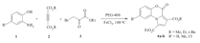
Our continued efforts to develop efficient and practical methodologies for the synthesis of novel N-containing heterocyclic compounds have led to the development of tandem reactions for the synthesis of heterocyclic compounds [11,12,13,14]. In this paper,we would like to disclose our discoveries on the iron(III) chloridecatalyzed tandem reaction of 2-aminophenols (1) with dialkyl acetylenedicarboxylates (2) in the presence of ethyl bromopyruvate (3) for the synthesis of pyrrolo[2,1-c][1, 4]benzoxazines (Scheme 1).
2. ExperimentalThe reagents and solvents used in this work were obtained from Aldrich and Fluka and were used without further purifications. Melting points were determined using an Electrothermal-9100 apparatus in open glass capillaries. IR spectra,in cm-1,were recorded on a Shimadzu IR-460 spectrometer using potassium bromide pellets. 1H NMR,and 13C NMR spectra were obtained at 250.1 and 75 MHz with a Bruker DRX-250 Avance instrument in CDCl3,δ in ppm,and J in Hz,and TMS used as an internal standard. MS spectra were measured using a Finnigan-MAT-8430 mass spectrometer at 70 eV,in m/z (rel.%). Elemental analyses were performed using a Heraeus CHN-O-rapid analyzer.
General procedures for the synthesis of pyrrolo[2,1-c][1, 4]benzoxazines 4: To a stirred solution of 1 (2 mmol) and 2 (2 mmol) in PEG-400 (0.5 mL) was added 3 (2 mmol) in the presence of FeCl3 (20 mol%) at room temperature. The resulting mixture was heated to 100℃. After the completion of the reaction (1 h),as indicated by TLC analysis (EtOAc/hexane,1:3),the reaction mixture was cooled to room temperature and then poured into 2 mL of water. The aqueous phase was extracted with CH2Cl2 (3 × 5 mL). The solvent was evaporated and the residue was purified by column chromatography using n-hexane-EtOAc (3:1) as eluent. The solvent was removed and the product was obtained.
4-Oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid, 1-ethyl ester,3-methyl ester (4a): Brown powder,mp: 158- 160℃,0.57 g,yield 91%. IR (KBr,cm-1): Vmax 1735 (C=O),1728 (C=O),1705 (C=O),1282 (C-O) cm-1. 1HNMR(250.1 MHz,CDCl3): δ 1.37 (t,3H,3J = 7.0 Hz,CH3),4.02 (s,3H,OCH3),4.35 (q,2H, 3J = 7.0 Hz,OCH2),7.37-7.42 (m,3H,3CH),7.66 (δ,1H,3J = 7.2 Hz, CH),8.12 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.1 (CH3),53.1 (OCH3),61.3 (OCH2),114.8 (CH),118.8 (CH),120.6 (CH),121.2 (C), 125.0 (C),125.1 (CH),127.6 (C),128.2 (CH),128.8 (C),143.3 (C), 151.9 (C=O),161.7 (C=O),164.1 (C=O). EI-MS: 315 (M+,27),256 (34),212 (34),149 (55),69 (76),57 (100). Anal. calcd. for C16H13NO6: C,60.95; H,4.16; N,4.44. Found: C,60.88; H,4.21; N, 4.49%.
4-Oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid, diethyl ester (4b): Brown powder,mp: 245℃ (dec.),0.59 g, yield 90%. IR (KBr,cm-1): Vmax 1733 (C=O),1726 (C=O),1695 (C=O),1280 (C-O) cm-1. 1HNMR (250.1 MHz,CDCl3): δ 1.18 (t,3H, 3J = 7.1 Hz,CH3),1.41 (t,3H,3J = 7.1 Hz,CH3),3.92 (q,2H, 3J = 7.1 Hz,OCH2),4.34 (q,2H,3J = 7.1 Hz,OCH2),7.32-7.37 (m, 3H,3CH),7.71 (δ,1H,3J = 7.3 Hz,CH),8.16 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.2 (CH3),14.5 (CH3),60.6 (OCH2),61.2 (OCH2),115.1 (CH),118.6 (CH),120.9 (CH),121.1 (C),125.2 (C), 125.5 (CH),127.4 (C),128.1 (CH),128.8 (C),143.4 (C),160.2 (C=O), 162.3 (C=O),164.6 (C=O). Anal. calcd. for C17H15NO6: C,62.00; H, 4.59; N,4.25. Found: C,61.88; H,4.51; N,4.29%.
4-Oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid, 3-t-butyl ester,1-ethyl ester (4c): Brown powder,mp: 195℃ (dec.),0.61 g,yield 86%. IR (KBr,cm-1): Vmax 1728 (C=O),1720 (C=O),1706 (C=O),1279 (C-O) cm-1. 1HNMR(250.1 MHz,CDCl3): δ 1.27 (s,9H,CMe3),1.38 (t,3H,3J = 7.1 Hz,CH3),4.25 (q,2H, 3J = 7.1 Hz,OCH2),7.28-7.34 (m,3H,3CH),7.59 (δ,1H,3J=7.2 Hz, CH),8.08 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.5 (CH3),27.4 (CMe3),61.3 (OCH2),81.6 (CMe3),115.2 (CH),118.6 (CH),120.7 (CH),121.1 (C),125.0 (C),125.4 (CH),127.6 (C),128.2 (CH),130.2 (C),142.8 (C),160.1 (C=O),162.1 (C=O),164.4 (C=O). Anal. calcd. for C19H19NO6: C,63.86; H,5.36; N,3.92. Found: C,63.81; H,5.41; N,3.92%.
8-Methyl-4-oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid,1-ethyl ester,3-methyl ester (4d): Brown powder, mp: 275-276℃,0.62 g,yield 94%. IR (KBr,cm-1): Vmax 1741 (C=O),1725 (C=O),1710 (C=O),1287 (C-O) cm-1. 1H NMR (250.1 MHz,CDCl3): δ 1.24 (t,3H,3J = 7.1 Hz,CH3),2.32 (s,3H, CH3),3.92 (s,3H,OCH3),4.25 (q,2H,3J = 7.1 Hz,OCH2),7.35-7.38 (m,2H,2CH),7.68 (s,1H,CH),8.14 (s,1H,CH). 13C NMR (62.9 MHz, CDCl3): δ 14.0 (CH3),21.4 (CH3),53.2 (OCH3),61.7 (OCH2),114.9 (CH),118.5 (CH),120.2 (CH),121.3 (C),124.9 (C),125.2 (C),127.6 (C),128.6 (CH),129.3 (C),142.8 (C),151.8 (C=O),161.9 (C=O),164.2 (C=O). Anal. Calcd. for C17H15NO6: C,62.00; H,4.59; N,4.25. Found: C,61.92; H,4.52; N,4.29%.
8-Methyl-4-oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid,diethyl ester (4e): Brown powder,mp: 256℃ (dec.), 0.63 g,yield 92%. IR (KBr,cm-1): Vmax 1736 (C=O),1729 (C=O), 1698 (C=O),1288 (C-O) cm-1. 1H NMR (250.1 MHz,CDCl3): δ 1.18 (t,3H,3J = 7.0 Hz,CH3),1.31 (t,3H,3J = 7.0 Hz,CH3),2.34 (s,3H, CH3),3.92 (q,2H,3J = 7.1 Hz,OCH2),4.25 (q,2H,3J = 7.1 Hz,OCH2), 7.35-7.38 (m,2H,2CH),7.74 (s,1H,CH),8.14 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.0 (CH3),14.6 (CH3),22.1 (CH3),60.9 (OCH2),61.4 (OCH2),115.4 (CH),118.2 (CH),120.6 (CH),121.4 (C), 124.5 (C),125.2 (C),127.1 (C),128.4 (CH),130.1 (C),141.4 (C), 152.4 (C=O),161.3 (C=O),164.5 (C=O). Anal. calcd. for C18H17NO6: C,62.97; H,4.99; N,4.08. Found: C,62.89; H,4.95; N,4.12%.
8-Methyl-4-oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid,3-t-butyl ester 1-ethyl ester (4f): Brown powder,mp: 195℃ (dec.),0.65 g,yield 88%. IR (KBr,cm-1): Vmax 1732 (C=O), 1718 (C=O),1702 (C=O),1280 (C-O) cm-1. 1H NMR (250.1 MHz, CDCl3): δ 1.30 (s,9H,CMe3),1.36 (t,3H,3J = 7.2 Hz,CH3),4.27 (q, 2H,3J = 7.2 Hz,OCH2),7.32-7.35 (m,2H,2CH),7.55 (s,1H,CH),8.08 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.4 (CH3),26.7 (CMe3), 61.5 (OCH2),81.1 (CMe3),114.9 (CH),118.5 (CH),120.2 (CH),121.8 (C),125.1 (C),125.5 (C),127.4 (C),128.0 (CH),129.6 (C),142.2 (C), 159.6 (C=O),161.7 (C=O),164.2 (C=O). Anal. Calcd. for C20H21NO6:C,64.68; H,5.70; N,3.77. Found: C,64.78; H,5.63; N,3.82%.
8-Chloro-4-oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid,1-ethyl ester,3-methyl ester (4g): Brown powder, mp: 269-271℃,0.62 g,yield 89%. IR (KBr,cm-1): Vmax 1736 (C=O), 1720 (C=O),1712 (C=O),1282 (C-O) cm-1. 1H NMR (250.1 MHz, CDCl3): δ 1.23 (t,3H,3J = 7.0 Hz,CH3),3.89 (s,3H,OCH3),4.27 (q, 2H,3J = 7.0 Hz,OCH2),7.32-7.37 (m,2H,2CH),7.69 (s,1H,CH),8.17 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.2 (CH3),52.5 (OCH3), 61.4 (OCH2),114.8 (CH),118.9 (CH),119.7 (CH),121.7 (C),124.2 (C),125.0 (C),128.1 (C),128.4 (CH),135.7 (C),141.3 (C),152.6 (C=O),162.3 (C=O),165.0 (C=O). Anal. calcd. for C16H12NO6: C, 54.95; H,3.46; N,4.01. Found: C,54.72; H,3.52; N,4.09%.
8-Chloro-4-oxo-4H-pyrrolo[2,1-c][1, 4]benzoxazine-1,3-dicarboxylic acid,diethyl ester (4h): Brown powder,mp: 247℃ (dec.), 0.63 g,yield 87%. IR (KBr,cm-1): Vmax 1740 (C=O),1732 (C=O), 1702 (C=O),1274 (C-O) cm-1. 1H NMR (250.1 MHz,CDCl3): δ 1.21 (t,3H,3J = 7.0 Hz,CH3),1.27 (t,3H,3J = 7.0 Hz,CH3),3.86 (q,2H, 3J = 7.0 Hz,OCH2),4.21 (q,2H,3J = 7.1 Hz,OCH2),7.41-7.46 (m,2H, 2CH),7.81 (s,1H,CH),8.19 (s,1H,CH). 13C NMR (62.9 MHz,CDCl3): δ 14.6 (CH3),14.9 (CH3),61.1 (OCH2),61.8 (OCH2),114.9 (CH), 118.5 (CH),121.0 (CH),121.4 (C),124.2 (C),124.9 (C),128.8 (C), 129.2 (CH),134.9 (C),141.2 (C),151.8 (C=O),162.4 (C=O),165.3 (C=O). Anal. calcd. for C17H14NO6: C,56.13; H,3.88; N,3.85. Found: C,56.09; H,3.91; N,3.92%. 3. Results and discussion
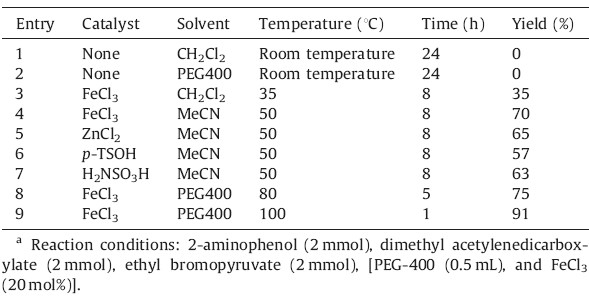
Our initial studies were focused on the reaction of ethyl bromopyruvate,2-aminophenol,and dimethyl acetylendicarboxylate for the synthesis of pyrrolo[2,1-c][1, 4]benzoxazine 4a. The reaction was investigated without the presence of catalyst,but we did not observe the desired product (Table 1,entries 1,2). It was found that 20 mol% of different catalyst such as iron(III) chloride and zinc chloride as Lewis acid or p-toluene sulfonic acid and sulfamic acid as Brønsted acid led to the formation of the targeted compound (Table 1,entries 4-7). According to these results,FeCl3 was selected as the optimal catalyst for the synthesis of pyrrolobenzoxazine 4a. Then different amount of this catalyst was examined in the model reaction. In the presence of 5,10,15,20 and 25% of FeCl3,the yield of pyrrolobenzoxazine 4a obtained was 45,60,78,91 and 91%,respectively. The best result was obtained with 20 mol% of the FeCl3 catalyst. Also the effects of the solvent and temperature were investigated. Under the optimized conditions, PEG-400 at 100℃ was found to be most favorable for high yields and short reaction times (Table 1,entry 9).
| Table 1 Optimization of the reaction conditionsa. |
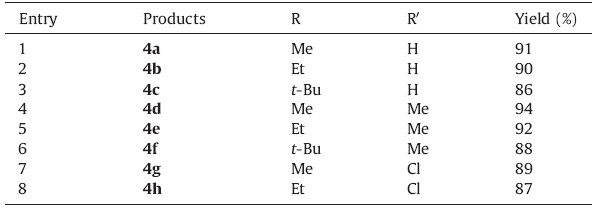
The 1H NMR and 13C NMR spectra of the crude products clearly indicated the formation of polysubstituted pyrrolo[2,1-c][1, 4]benzoxazine derivatives 4a-h in 86-94% yields (Table 2).
The structures of products 4a-h were characterized by 1H NMR, 13C NMR,IR spectra,and MS. For example,the mass spectrum of 4a displayed a molecular ion peak at m/z 315. The IR spectrum of 4a displayed the characteristic signals for carbonyl bonds (1735, 1728,and 1705 cm-1). The 1H NMR spectrum of 4a exhibited a signal at 4.02 ppm for the methoxy group,a quartet at 4.35 ppm, and a triplet at 1.37 ppm for the ethoxy group,and multiplets at 7.37-8.12 ppm for the aromatic rings. The proton-decoupled 13C NMR spectrum of 4a showed 16 distinct resonances in agreement with the proposed structure. The 1H NMR and 13C NMR spectra of products 4b-h were similar to those for 4a,except for the ester moieties,which exhibited characteristic resonances in the appropriate regions of the spectrum.
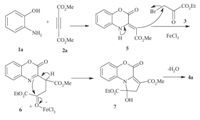
Based on these results,a possible mechanism for the formation of pyrrolo[2,1-c][1, 4]benzoxazines is shown in Scheme 2. The reaction between 2-aminophenol 1a and dimethyl acetylenedicarboxylate 2a affords benzoxazine 5. The resulting enamine reacts with the ethyl bromopyruvate 3 in the presence of FeCl3 to give intermediate 6,which undergoes a series of cyclization and elimination reactions to generate product 4a.
|
Download:
|
| Scheme 2.Proposed mechanism for the formation of compound 4a. | |
In summary,we have discovered a novel,mild,and straightforward procedure for the synthesis of a new class of substituted N-heterocyclic derivatives using the three-component reaction involving 2-aminophenols,dialkyl acetylenedicarboxylates,and ethyl bromopyruvate in the presence of 20 mol% FeCl3 in PEG-400. Depending on the 2-aminophenols and dialkyl acetylenedicarboxylates, various new pyrrolo[2,1-c][1, 4]benzoxazines have been prepared. The yields of the products,the availability of the starting materials,and the simplicity of the process render this protocol a useful method for the preparation of this ring system. Acknowledgment
We are grateful to Sanandaj Branch,Islamic Azad University Research Council for the financial support of this research.
| [1] | A. Dömling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39 (2000) 3168-3210. |
| [2] | V. Nair, R.S. Menon, V. Sreekumar, Multicomponent reactions based on nucleophilic carbenes and their applications in organic synthesis, Pure Appl. Chem. 77 (2005) 1191-1198. |
| [3] | A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106 (2006) 17-89. |
| [4] | G. Campiani, A. Garofalo, I. Fiorini, et al., Pyrrolo[2,1-c][1,4]benzothiazines: synthesis, structure-activity relationships, molecular modeling studies, and cardiovascular activity, J. Med. Chem. 38 (1995) 4393-4410. |
| [5] | D.S. Snyder, L. Tradtrantip, C. Yao, M.J. Kurth, A.S. Verkman, Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease, J. Med. Chem. 54 (2011) 5468-5477. |
| [6] | X.Q. Xie, X.W. Han, J.Z. Chen, M. Eissenstat, A. Makriyannis, High-resolution NMR and computer modeling studies of a highly potent cannabimimetic ligand aminoalkyl indoles, J. Med. Chem. 42 (1999) 4021-4027. |
| [7] | M. Artico, G. Porretta, G. De Martino, The synthesis of 4H-pyrrole[2,1-c][1,4]benzoxazine, J. Heterocycl. Chem. 6 (1971) 283-287. |
| [8] | G. Cheeseman, M. Rafiz, P.D. Roy, C. Turner, G.V. Boyd, Synthesis and reactions of pyrrolo[2,1-c][1,2,4]benzotriazine and 4-oxo-4H-pyrrolo[2,1-c][1,4]benzoxazine, J. Chem. Soc. C (1971) 2018-2022. |
| [9] | I. Sanchez, M.D. Pujol, A convenient synthesis of pyrrolo[21-c][1,4] benzoxazines, Tetrahedron 55 (1999) 5593-5598. |
| [10] | S. Kurhade, R.D. Kaduskar, B. Dave, et al., Synthesis of a novel tetracyclic azaindolo[2,1-c][1,4]benzoxazine ring system, Tetrahedron Lett. 52 (2011) 1874-1877. |
| [11] | I. Yavari, M. Piltan, L. Moradi, Synthesis of pyrrolo[2,1-a]isoquinolines from activated acetylenes, benzoylnitromethanes, and isoquinoline, Tetrahedron 65 (2009) 2067-2071. |
| [12] | M. Piltan, L. Moradi, H. Salimi, K. Zargoosh, S.A. Zarei, PEG-mediated catalyst-free expeditious synthesis of polysubstituted anilines and benzenes via the reaction of malononitrile and b-ketoester derivatives in the presence of activated acetylenes, Comb. Chem. High Throughput Screening 15 (2012) 571-575. |
| [13] | L. Moradi, M. Piltan, H. Rostami, G. Abasi, One-pot synthesis of pyrrolo[1,2- a]pyrazines via three component reaction of ethylenediamine, acetylenic esters and nitrostyrene derivatives, Chin. Chem. Lett. 24 (2013) 740-742. |
| [14] | M. Piltan, I. Yavari, L. Moradi, Tandem synthesis of functionalized hexaalkyl benzoisoquinolinopyrrolonaphthyridine-hexacarboxylate, via isoquinoline based multi-component reaction, Chin. Chem. Lett. 24 (2013) 979-983. |