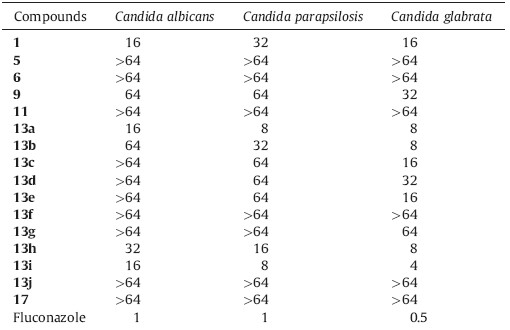
There is an urgent need for novel antifungal agents because the incidence of invasive fungal infections (IFIs) has been increasing dramatically [1,2]. Immunocompromised hosts,such as patients undergoing anticancer chemotherapy or organ transplants and patients with AIDS,are vulnerable populations for IFIs and the associated mortality is very high [3]. Candida albicans is the most common cause of IFIs,which ranks the third to fourth among the bloodstream infections in the United States [2]. However,effective and safe antifungal agents that can be used for life-threatening fungal infections are very limited. Clinically,four classes of antifungal agents (Fig. 1),namely amphotericin B [4],azoles (e.g. fluconazole and itraconazole) [5],echinocandins (e.g. caspofungin and micafungin) [6] and 5-fluorocytosine,are available for the treatment of IFIs. Unfortunately,they have had only limited success in reducing the high mortality rate of IFIs. Moreover,severe resistance of antifungal drugs is also becoming a serious problem [7]. Therefore,there is a pressing need to develop novel antifungal agents with new chemical scaffold and fungal-specific mechanisms [8].
In our previous studies,tetrahydrocarbazole derivatives (Fig. 1) were identified as antifungal leads [9],which provide a good starting point for structural optimization. Continuing our efforts in antifungal drug discovery [10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23],the structure-activity relationship (SAR) of the tetrahydrocarbazole lead was extended and several targeted compounds showed potent activity against Candida species.
|
Download:
|
| Fig. 1.Chemical structures of representative antifungal agents and the lead compound 1. | |
All starting materials were commercially available and analytically pure. 1H NMR spectra were recorded on a BRUKER AVANCE 500 spectrometer (Bruker Company,Germany),using TMS as an internal standard and CDCl3 as solvents. Chemical shifts (δ values) and coupling constants (J values) are given in ppm and Hz,respectively. TLC analysis was carried out on silica gel plates GF254 (Qindao Haiyang Chemical,China).
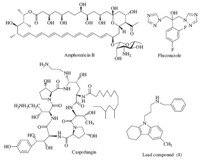
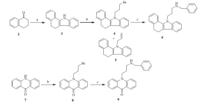
Synthetic routes of the targeted compounds are outlined in Schemes 1-2. According to the procedure of Fisher indole synthesis,3,4-dihydronaphthalen-1(2H)-one (2) was condensed with phenylhydrazine hydrochloride in EtOH to give the annulated benzocarbazole 3 [24]. Then,it was alkylated by 1,3-dibromopropane using KOH as a base. Notably,two products,namely substituted compound 4 and eliminated compound 5,were obtained in this reaction. A reaction of intermediate 4 with benzyl amine in the presence of K2CO3 and DMF gave the targeted compound 6. Using acridone and carbazole as the starting material, targeted compounds 9 and 13a-j were prepared using a similar procedure in moderate to good yields. Intermediate 14 was obtained by substituting carbazole 10 with 3-bromoprop-1-yne [25],which was subsequently reacted with (azidomethyl)benzene (16) using the classical conditions of the click reaction to afford the targeted compound 17.
|
Download:
|
| Scheme 1.Synthetic route of compounds 4-9. Reagents and conditions: (a) phenylhydrazine hydrochloride,EtOH,reflux,4 h,69.1%; (b) 1,3-dibromopropane,KOH,DMSO,r.t.,3 h,27.0%-38.5%; (c) benzyl amine,K2CO3,DMF,80℃,4 h,16.4%-67.6%. | |
|
Download:
|
| Scheme 2.Synthetic route of compounds 11-17. Reagents and conditions: (a) 1,3-dibromopropane,KOH,DMSO,r.t.,3 h,32.4%-43.7%; (b) benzyl amine,K2CO3,DMF,80℃,4 h,16.4%-67.6%; (c) 3-bromoprop-1-yne,KOH,r.t.,4 h,82.4%; (d) sodium azide,DMSO,r.t.,12 h,100%; (e) VcNa,CuSO4,DMSO/H2O,r.t.,24 h,89.6%. | |
To a solution of intermediate 3 (2.19 g,0.01 mol) and 1,3- dibromopropane (8.08 g,0.04 mol,4 equiv.) in DMSO (50 mL) was added KOH (2.24 g,0.04 mol,4 equiv.) and the mixture was allow to stir at ambient temperature for 3 h. Then,the reaction mixture was diluted with water (50 mL) and extracted with diethyl ether (50 mL × 3). The combined organic layer was washed with brine (100 mL),dried over anhydrous MgSO4,filtered,and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc,40:1,v/v) to give compound 4 as a white solid (1.31 g,38.5% yield). 1H NMR (500 Hz,CDCl3): δ 7.13-7.59 (m,8H,Ar-H),4.59-4.63 (m,2H,NCH2CH2CH2Br),3.40 (t,2H,J = 6.2 Hz,NCH2CH2CH2Br),2.95-2.99 (m,2H,carbazole-3- CH2),2.88-2.92 (m,2H,carbazole-4-CH2),2.40-2.45 (m,2H, NCH2CH2CH2Br).
Compound 5 (11-allyl-6,11-dihydro-5H-benzo[a]carbazole)was the by-product in the synthesis of intermediate 4. White solid (0.70 g,27.0% yield). 1H NMR (500 Hz,CDCl3): δ 7.05-7.57 (m,8H,Ar-H),6.16-6.21 (m,1H,allyl-2-CH),5.21 (δ,1H,J = 10.5 Hz,allyl-3- Ha),5.03 (s,2H,allyl-CH2),4.87 (δ,1H,J = 17.0 Hz,allyl-3-Hb),2.90- 2.94 (m,2H,CH2CH2),2.83-2.87 (m,2H,CH2CH2). 2.2. Synthesis of 3-(5H-benzo[a]carbazol-11(6H)-yl)-Nbenzylpropan-1-amine (6)
To a solution of intermediate 4 (0.34 g,1 mmol,1 equiv.) and phenylmethanamine (0.86 g,8 mmol,8 equiv.) in DMF (10 mL) was added K2CO3 (0.28 g,2 mmol,2 equiv.) and the mixture was allowed to stir at 80℃ for 4 h. Then,the reaction mixture was diluted with diethyl ether (25 mL) and washed with water (10 mL × 3). The organic layer was separated,dried over Na2SO4, filtered,and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc,40:1,v/v) to give the targeted compound 6 as a pale yellow solid (0.06 g,16.4% yield). 1H NMR (500 Hz,CDCl3): δ 7.12-7.68 (m,13H,Ar-H),4.55 (t, 2H,J = 7.5 Hz,NCH2CH2CH2NH),3.76 (s,2H,phenyl-CH2),2.90- 3.01 (m,4H,carbazole-3,4-CH2),2.70 (t,2H,J = 6.8 Hz, NCH2CH2CH2NH),2.07-2.19 (m,2H,NCH2CH2CH2NH); MS (ESI)m/z: 367 (M+1).
The synthetic methods for the targeted compounds 8-13 were similar to the above procedure.
10-(3-Bromopropyl)acridin-9(10H)-one (8): Yellow solid (1.01 g, 32.0% yield). 1H NMR (500 Hz,CDCl3): δ 7.32-8.37 (m,8H,Ar-H), 4.81 (t,2H,J = 7.8 Hz,NCH2CH2CH2Br),3.86 (t,2H,J = 6.0 Hz, NCH2CH2CH2Br),2.63-2.69 (m,3H,NCH2CH2CH2Br).
10-(3-(Benzylamino)propyl)acridin-9(10H)-one (9): Yellow solid (0.23 g,67.6% yield). 1H NMR (500 Hz,CDCl3): δ 7.32-8.37 (m,13H, Ar-H),4.57 (t,2H,J = 7.7 Hz,NCH2CH2CH2NH),3.75 (s,2H,phenyl- CH2),2.72 (t,2H,J = 6.3 Hz,NCH2CH2CH2Br),1.95-1.99 (m,2H, NCH2CH2CH2NH); MS (ESI) m/z: 343 (M+1).
9-Allyl-9H-carbazole (11): White solid (0.67 g,32.4% yield). 1H NMR (500 Hz,CDCl3): δ 7.18-8.17 (m,8H,Ar-H),5.91-6.01 (m,1H, allyl-2-CH),5.10 (dd,1H,J1 = 10.1 Hz,J2 = 1.4 Hz,allyl-3-Ha),5.04 (δ,2H,J = 5.1 Hz,allyl-CH2),4.96 (dd,1H,J1 = 17.1 Hz,J2 = 1.5 Hz, allyl-3-Hb).
9-(3-Bromopropyl)-9H-carbazole (12): White solid (1.26 g,43.7% yield). 1H NMR (500 Hz,CDCl3): δ 7.23-8.13 (m,8H,Ar-H),4.52 (t, 2H,J = 6.5 Hz,NCH2CH2CH2Br),3.40 (t,2H,J = 6.3 Hz, NCH2CH2CH2Br),2.41-2.50 (m,2H,NCH2CH2CH2Br).
N-Benzyl-3-(9H-carbazol-9-yl)propan-1-amine (13a): White solid (0.15 g,48.4% yield). 1H NMR (500 Hz,CDCl3): δ 7.17-8.15(m,13H,Ar-H),4.46 (t,2H,J = 6.9 Hz,NCH2CH2CH2NH),3.65 (s,2H, phenyl-CH2),3.29 (br,2H,NCH2CH2CH2NH),1.91-1.95 (m,2H, NCH2CH2CH2NH); MS (ESI) m/z: 315 (M+1).
3-(9H-Carbazol-9-yl)-N-(4-fluorobenzyl)propan-1-amine (13b): 1H NMR (500 Hz,CDCl3): δ 7.09-8.15 (m,12H,Ar-H),4.45 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.61 (s,2H,phenyl-CH2),2.48 (t, 2H,J = 6.5 Hz,NCH2CH2CH2NH),2.26 (br,1H,NH),1.88-1.94 (m, 2H,NCH2CH2CH2NH); MS (ESI) m/z: 333 (M+1).
3-(9H-Carbazol-9-yl)-N-(3-fluorobenzyl)propan-1-amine (13c): 1H NMR (500 Hz,CDCl3): δ 7.13-8.15 (m,12H,Ar-H),4.46 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.65 (s,2H,phenyl-CH2),2.49 (br, 2H,NCH2CH2CH2NH),2.30 (br,1H,NH),1.90-1.93 (m,2H, NCH2CH2CH2NH); MS (ESI) m/z: 333 (M+1).
3-(9H-Carbazol-9-yl)-N-(2-fluorobenzyl)propan-1-amine (13d): 1H NMR (500 Hz,CDCl3): δ 7.11-8.15 (m,12H,Ar-H),4.46 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.68 (s,2H,phenyl-CH2),2.51 (br, 2H,NCH2CH2CH2NH),2.26 (br,1H,NH),1.89-1.95 (m,2H, NCH2CH2CH2NH); MS (ESI) m/z: 333 (M+1).
3-(9H-Carbazol-9-yl)-N-(4-chlorobenzyl)propan-1-amine (13e): 1H NMR (500 Hz,CDCl3): δ 7.17-8.15 (m,12H,Ar-H),4.45 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.62 (s,2H,phenyl-CH2),2.49 (br, 2H,NCH2CH2CH2NH),1.90-1.93 (m,2H,NCH2CH2CH2NH); MS (ESI) m/z: 349 (M+1).
3-(9H-Carbazol-9-yl)-N-(2-chlorobenzyl)propan-1-amine (13f): 1H NMR (500 Hz,CDCl3): δ 7.17-8.15 (m,12H,Ar-H),4.46 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.74 (s,2H,phenyl-CH2),2.54 (t, 2H,J = 7.2 Hz,NCH2CH2CH2NH),1.90-1.93 (m,2H, NCH2CH2CH2NH); MS (ESI) m/z: 349 (M+1).
3-(9H-Carbazol-9-yl)-N-(3-chlorobenzyl)propan-1-amine (13g): 1H NMR (500 Hz,CDCl3): δ 7.17-8.15 (m,12H,Ar-H),4.47 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.65 (s,2H,phenyl-CH2),2.49 (br, 2H,NCH2CH2CH2NH),1.89-1.95 (m,2H,NCH2CH2CH2NH); MS (ESI) m/z: 349 (M+1).
3-(9H-Carbazol-9-yl)-N-(4-methylbenzyl)propan-1-amine (13h): 1H NMR (500 Hz,CDCl3): δ 7.08-8.15 (m,12H,Ar-H),4.45 (t,2H, J = 6.9 Hz,NCH2CH2CH2NH),3.58 (s,2H,phenyl-CH2),2.48 (br,2H, NCH2CH2CH2NH),2.27 (s,3H,CH3),1.89-1.92 (m,2H, NCH2CH2CH2NH); MS (ESI) m/z: 329 (M+1).
3-(9H-Carbazol-9-yl)-N-(4-methoxybenzyl)propan-1-amine (13i): 1H NMR (500 Hz,CDCl3): δ 6.84-8.15 (m,12H,Ar-H),4.45 (t, 2H,J = 6.9 Hz,NCH2CH2CH2NH),3.72 (s,3H,OCH3),3.56 (s,2H, phenyl-CH2),2.48 (t,2H,J = 6.9 Hz,NCH2CH2CH2NH),1.89-1.92 (m,2H,NCH2CH2CH2NH); MS (ESI) m/z: 345 (M+1).
3-(9H-Carbazol-9-yl)-N-(2,4-dichlorobenzyl)propan-1-amine (13j): 1H NMR (500 Hz,CDCl3): δ 7.71-8.71 (m,11H,Ar-H),5.02 (t, 2H,J = 6.6 Hz,NCH2CH2CH2NH),4.26 (s,2H,phenyl-CH2),3.06 (br, 2H,NCH2CH2CH2NH),2.46-2.51 (m,2H,NCH2CH2CH2NH); MS(ESI) m/z: 384 (M+1). 2.3. Synthesis of 9-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-9Hcarbazole(17)
A suspension of (bromomethyl)benzene (0.86 g,5.0 mmol, 5 equiv.) and NaN3 (0.36 g,5.5 mmol,5.5 equiv.) in DMSO (25 mL) was allowed to stir overnight. Then,intermediate 14 (0.20 g, 1.0 mmol,1 equiv.),an aqueous solution of sodium ascorbate (0.01 mol/L,10 mL,0.1 equiv) and a copper sulfate solution (0.005 mol/L,2 mL,0.01 equiv) were added under a nitrogen atmosphere and was allowed to stir at ambient temperature for 24 h. The reaction mixture was diluted with water (20 mL) and extracted with EtOAc (20 mL × 3). The combined organic layer was washed with brine (30 mL),dried over anhydrous MgSO4,filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane/EtOAc,4:1,v/v) to give the targeted compound 17 as a white solid (0.60 g,89.6% yield). 1HNMR (500 Hz,CDCl3): δ 7.18-8.15 (m,14H,Ar-H),5.66 (s,2H, phenyl-CH2),5.49 (s,2H,carbazole-CH2). MS (ESI) m/z: 339 (M+1). 2.4. In vitro antifungal activity assays
The in vitro antifungal activity was measured by means of the MIC using the serial dilution method in 96-well microtest plates. Test fungal strains were obtained from the ATCC or were clinical isolates. The MIC determination was performed according to the National Committee for Clinical Laboratory Standards (NCCLS) recommendations using RPMI 1640 (Sigma) buffered with MOPS (3-morpholinopropanesulfonic acid,0.165 mol/L,Sigma) as the test medium. The MIC value was defined as the lowest concentration of test compounds that resulted in a culture with turbidity less than or equal to 80% inhibition when compared to the growth of the control. Test compounds were dissolved in DMSO serially diluted in growth medium. Plates were incubated without shaking at 35℃. Optical density (OD) was measured at 630 nmand the background OD was subtracted from each well. Growth MIC was determined at 24 h for Candida species. Each experiment was performed in triplicate. 3. Results and discussion
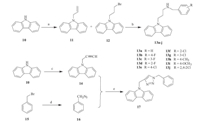
The design rationale for the targeted compounds is depicted in Fig. 2. The tetrahydrocarbazole scaffold was replaced by benzocarbazole, acridone and carbazole,respectively. Two kinds of side chain,namely benzylaminopropyl and benzyltriazolmethyl,were also investigated. In vitro antifungal activity of the targeted compounds was expressed as the minimal inhibitory concentration (MIC) that achieved 80% inhibition of the tested fungi. Three kinds of pathogenic fungi,namely Candida albicans,Candida parapsilosis and Candida glabrata,were chosen for assaying because Candidosis is the leading cause of the IFIs. Fluconazole was used as a positive control.
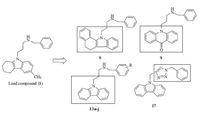
|
Download:
|
| Fig. 2.Design rationale of the targeted compounds. | |
Initially,we aimed to investigate the influence of the tetrahydrocarbazole scaffold on the antifungal activity. When the tetrahydrocarbazole core was annulated with a phenyl group, the resulting compound 6 was found to be inactive. If the tetrahydrocarbazole scaffold was replaced by the acridone,the antifungal activity of compound 9 decreased (MIC range: 16- 32 mg/mL). Interestingly,the aromatization of the tetrahydrocarbazole core to carbazole led to an increase of the antifungal activity. Compound 13a showed potent activity against Candida albicans,Candida parapsilosis,Candida glabrata with MIC values of 16 mg/mL,16 mg/mL and 8 mg/mL,respectively. The results indicated that the scaffold of this series of compounds played an important role for the antifungal activity.
Then,various substitutions were introduced on the terminal phenyl group (compounds 13a-j in Table 1). When position 4 of the phenyl group was substituted with a fluorine (compound 13b) or chlorine (compound 13e) atom,their antifungal activity generally decreased. Moving the fluorine or chlorine substituent to position 2 or 3 resulted in further decrease of the antifungal activity. The results revealed that halogen substitutions on the phenyl group were not favorable for the antifungal activity. On the other hand,the introduction of 4-methyl and 4-methoxyl group on compound 13a led to comparable or improved antifungal activity. Particularly,compound 13i (MIC = 4 mg/mL) showed better inhibitory activity against Candida glabrata than compound 13a (MIC = 8 mg/mL). In order to investigate the importance the terminal benzyl group,two by-products from the elimination reaction,5 and 11,were also subjected to antifungal assaying. Unfortunately,they are totally inactive,indicating that the terminal benzyl group was essential for the antifungal activity. Moreover,1,2,3-triazole was also designed as a linker in the side chain. However,the loss of the antifungal activity was observed for compound 17,highlighting the importance of the benzylaminopropyl side chain. Notably,these carbazole derivatives were designed from N-myristoyltransferase (NMT) inhibitors [9],the determination of their NMT inhibitory activity would be helpful to clarify the mode of action and provide important information for further structural optimization.
| Table 1 In vitro antifungal activities of the carbazole derivatives (MIC80,mg/mL). |
In summary,a series of novel carbazole derivatives were designed and synthesized as antifungal lead compounds. The SARs revealed that the carbazole scaffold was better than tetrahydrocarbazole and the benzylaminopropyl side chain was important for the antifungal activity. The introduction of 4-methoxyl substituent slightly improved the antifungal activity. Compound 13i showed better antifungal activity than the lead compound 1. Although it was still less active than fluconazole,it can be used as a good starting point for further optimization. Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (No. 81222044),the 863 Hi-Tech Program of China (No. 2012AA020302),Shanghai Rising-Star Program (No. 12QH1402600),and Shanghai Municipal Health Bureau (No. XYQ2011038).
| [1] | M.A. Pfaller, D.J. Diekema, Epidemiology of invasive candidiasis: a persistent public health problem, Clin. Microbiol. Rev. 20 (2007) 133-163. |
| [2] | L. Ostrosky-Zeichner, A. Casadevall, J.N. Galgiani, et al., An insight into the antifungal pipeline: selected new molecules and beyond, Nat. Rev. Drug Discov. 9 (2010) 719-727. |
| [3] | S.K. Fridkin, W.R. Jarvis, Epidemiology of nosocomial fungal infections, Clin. Microbiol. Rev. 9 (1996) 499-511. |
| [4] | H.A. Gallis, R.H. Drew, W.W. Pickard, Amphotericin B: 30 years of clinical experience, Rev. Infect. Dis. 12 (1990) 308-329. |
| [5] | D.J. Sheehan, C.A. Hitchcock, C.M. Sibley, Current and emerging azole antifungal agents, Clin. Microbiol. Rev. 12 (1999) 40-79. |
| [6] | D.W. Denning, Echinocandins: a new class of antifungal, J. Antimicrob. Chemother. 49 (2002) 889-891. |
| [7] | I.A. Casalinuovo, P. Di Francesco, E. Garaci, Fluconazole resistance in Candida albicans: a review of mechanisms, Eur. Rev. Med. Pharmacol. Sci. 8 (2004) 69-77. |
| [8] | C. Sheng, W. Zhang, New lead structures in antifungal drug discovery, Curr. Med. Chem. 18 (2011) 733-766. |
| [9] | W. Wang, G. Dong, J. Gu, et al., Structure-activity relationships of tetrahydrocarbazole derivatives as antifungal lead compounds, Med. Chem. Commun. 4 (2013) 353-362. |
| [10] | X. Che, C. Sheng, W. Wang, et al., New azoles with potent antifungal activity: design, synthesis and molecular docking, Eur. J. Med. Chem. 44 (2009) 4218- 4226. |
| [11] | Z. Jiang, Y. Wang, W. Wang, et al., Discovery of highly potent triazole antifungal derivatives by heterocycle-benzene bioisosteric replacement, Eur. J. Med. Chem. 64C (2013) 16-22. |
| [12] | Z. Lv, C. Sheng, Y. Zhang, et al., Synthesis and in vitro antifungal activities of new 3- substituted benzopyrone derivatives, Bioorg. Med. Chem. Lett. 20 (2010) 7106- 7109. |
| [13] | C. Sheng, X. Che, W. Wang, et al., Design and synthesis of novel triazole antifungal derivatives by structure-based bioisosterism, Eur. J. Med. Chem. 46 (2011) 5276- 5282. |
| [14] | C. Sheng, X. Che, W. Wang, et al., Structure-based design, synthesis, and antifungal activity of new triazole derivatives, Chem. Biol. Drug. Des. 78 (2011) 309-313. |
| [15] | C. Sheng, X. Che, W. Wang, et al., Design and synthesis of antifungal benzoheterocyclic derivatives by scaffold hopping, Eur. J. Med. Chem. 46 (2011) 1706- 1712. |
| [16] | C. Sheng, S. Chen, H. Ji, et al., Evolutionary trace analysis of CYP51 family: implication for site-directed mutagenesis and novel antifungal drug design, J. Mol. Model. 16 (2010) 279-284. |
| [17] | C. Sheng, H. Xu, W. Wang, et al., Design, synthesis and antifungal activity of isosteric analogues of benzoheterocyclic N-myristoyltransferase inhibitors, Eur. J. Med. Chem. 45 (2010) 3531-3540. |
| [18] | C. Sheng, W. Zhang, H. Ji, et al., Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking, J. Med. Chem. 49 (2006) 2512- 2525. |
| [19] | W. Wang, C. Sheng, X. Che, et al., Discovery of highly potent novel antifungal azoles by structure-based rational design, Bioorg. Med. Chem. Lett. 19 (2009) 5965-5969. |
| [20] | W. Wang, C. Sheng, X. Che, et al., Design, synthesis, and antifungal activity of novel conformationally restricted triazole derivatives, Arch Pharm. (Weinheim) 342 (2009) 732-739. |
| [21] | W. Wang, S. Wang, Y. Liu, et al., Novel conformationally restricted triazole derivatives with potent antifungal activity, Eur. J. Med. Chem. 45 (2010) 6020- 6026. |
| [22] | Y. Xu, C. Sheng, W. Wang, et al., Structure-based rational design, synthesis and antifungal activity of oxime-containing azole derivatives, Bioorg. Med. Chem. Lett. 20 (2010) 2942-2945. |
| [23] | J. Yao, H. Liu, T. Zhou, et al., Total synthesis and structure-activity relationships of new echinocandin-like antifungal cyclolipohexapeptides, Eur. J. Med. Chem. 50 (2012) 196-208. |
| [24] | M. Desroses, K. Wieckowski, M. Stevens, et al., A microwave-assisted, propylphosphonic anhydride (T3P.(R).) mediated one-pot Fischer indole synthesis, Tetrahedron Lett. 52 (2011) 4417-4420. |
| [25] | H. Gan, H. Liu, Y. Li, et al., Fabrication of polydiacetylene nanowires by associated self-polymerization and self-assembly processes for efficient field emission properties, J. Am. Chem. Soc. 127 (2005) 12452-12453. |