Macrocyclic compounds,specifically calixarenes,have become a popular building block for the preparation of fluorescent sensors [1, 2, 3]. Calix[4]arene is widely used as a molecular scaffold in the design of fluorescent receptors because of its tunable and unique three-dimensional structure in addition to the ease of functionalization. For example,lower rim functionalization can be achieved quite easily by etherification of the phenolic OH groups [4, 5, 6]. However,it is still challenging to effectively introduce functional groups to calix[4]arenes with fluorescence characteristics. The palladium-catalyzed cross-coupling reactions play a vital role in fluorescent calixarene syntheses because they provide an effective way to combine the ionophores and fluorophores. For example, Georghiou and co-workers reported functionalizing the narrow rim of calix[4]arenes using metal-assisted coupling reactions,such as the Stille and Suzuki-Miyaura reactions on the corresponding calix[4]arene triflates or mesylates [7, 8, 9]. Gonza´ lez et al. also synthesized fascinating calixarenes using the Pd-catalyzed Stille reaction [10, 11]. At the same time the Pd-catalyzed Sonogashira reaction has already been used successfully to prepare interesting calixarenes.
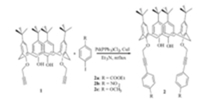
Herein,we report the synthesis of a series of functionalized calixarenes and the fluorescence properties of a calix[4]arene (2c) towards phenol derivatives. The synthesis of the target compounds 2 (2a-c) was depicted in Scheme 1. The double Sonogashira crosscoupling reaction is described as an efficient synthetic tool for the preparation of calixarenes with relatively deep electron-rich cavities. Iodobenzene can be coupled with different substituents containing terminal alkyne calix[4]arene successfully and efficiently. Reactions of halocalixarenes with terminal alkynes led to the coupling products in yields up to 80%. Subsequently,we minimized the free energy of the complexes by theoretical calculations. The π-π stacking interactions took place between the 4-nitrophenol and calix[4]arenes,which may lead to the significant fluorescence quench.
|
Download:
|
| Scheme 1.Synthesis of calix[4]arene derivatives via Sonogashira reaction. | |
1H NMR and 13C NMR were recorded on a Varian Mercury VX400 instrument at ambient temperature using TMS as an internal standard. ESI-MS data were recorded on a Finnigan LCQ-Advantage instrument and melting points were recorded on a METTLER TOLEDO FP62 instrument. All reagents were commercially available and purified by standard methods prior to use. The starting material compound 1 was prepared using the reported procedures [12, 13] and p-iodobenzene derivatives and bis-(triphenylphosphine)palladium(II) dichloride were purchased. 2.1. General procedures for the synthesis of calix[4]arene derivatives
To a stirred mixture of Pd(PPh3)2Cl2 (28 mg,0.04 mmol),CuI (9 mg,0.04 mmol) in triethylamine (20 mL) was added a solution of 1 (0.145 g,0.2 mmol) in triethylamine (30 mL) at reflux temperature under the nitrogen. The reaction mixture was stirred for 18 h. The solvent was evaporated using a rotary evaporator and the resulting crude product was dissolved in CH2Cl2 and washed with saturated brine. The CH2Cl2 extracts were dried (MgSO4) and the solvent was removed under vacuum to give the crude product, which was purified by TLC (10:1 PE/EA) to give the following compounds.
Compound 2a: Yield 81%,mp 162.1℃,1H NMR (600 MHz, CDCl3): δ 0.94 (s,18H,t-butyl),1.33 (s,18H,t-butyl),1.38 (t,6H, CH3),3.35 (d,4H,J = 13.8 Hz,ArCH2Ar),4.42 (d,4H,J = 13.8 Hz, ArCH2Ar),4.45 (m,4H,CH2CH3),5.01 (s,4H,OCH2),6.67 (s,2H, ArOH),6.77 (s,4H,ArH),7.08 (s,4H,ArH),7.45 (d,4H,J = 7.8 Hz, ArH),7.98 (d,4H,J = 7.2 Hz,ArH). ESI-MS (m/z): 1043.6 [M+Na+]. Anal. calcd. for C68H76O8: C 79.97,H7.50. Found: C 79.94,H,7.52.
Compound 2b: Yield 95%,mp 182.6℃,1H NMR (400 MHz, CDCl3): δ 0.92 (s,18H,t-butyl),1.31 (s,18H,t-butyl),3.36 (d,4H, J = 12.6 Hz,ArCH2Ar),4.43 (d,4H,J = 13.2 Hz,ArCH2Ar),5.02 (s,4H, OCH2),6.67 (s,2H,ArOH),6.77 (s,4H,ArH),7.09 (s,4H,ArH),7.54 (d,4H,J = 9 Hz,ArH),8.18 (d,4H,J = 8.4 Hz,ArH). 13C NMR (100 MHz,CDCl3): δ 150.3,149.3,147.6,141.9,132.6,132.4,127.9, 125.6,125.1,123.6,89.7,86.2,63.8,33.9,32.0,31.7,30.9; ESI-MS (m/z): 990.5 [M+Na+]. Anal. calcd. for C62H66N2O8: C 76.99,H 6.88, N 2.90. Found: C 77.19,H 7.01,N 3.09.
Compound 2c: Yield 72%,1H NMR (400 MHz,CDCl3): δ 0.92 (s, 18H,t-butyl),1.30 (s,18H,t-butyl),3.32 (d,4H,J = 14.4 Hz, ArCH2Ar),3.90 (s,6H,OCH3),4.42 (d,4H,J = 14.4 Hz,ArCH2Ar), 4.96 (s,4H,OCH2),6.65 (s,2H,ArOH),6.75 (s,4H,ArH),7.06 (s,4H, ArH),7.11 (d,4H,J = 12.6 Hz,ArH),7.63 (d,4H,J = 12.6 Hz,ArH). ESI-MS (m/z): 959.5 [M+Na+]. Anal. calcd. for C64H72O6: C 82.01,H 7.74. Found: C 82.25,H 7.97. 2.2. General procedures for the recognition of phenols
Calix[4]arene (2c) was dissolved in acetonitrile to prepare a stock solution with a concentration of 5.0 × 10-6 mol/L for the fluorescent spectroscopy measurement. The guests are the phenol derivatives (d1-d8),including p-nitrophenol,o-nitrophenol,omethylphenol, p-methylphenol,phenol,catechol,resorcinol,and hydroquinol. The fluorescent spectra were recorded on a Cary Eclipse FL1008M018 instrument. 3. Results and discussion
Calix[4]arene derivatives can be obtained in excellent yields by the palladium-catalyzed cross-coupling reactions. The reaction of aryl iodide bearing an electron-donating methoxy group permit the cross-coupling with calix[4]arene 1,giving the desired product 2c in 72% yield. Aryl iodides bearing electron-withdrawing groups such as -COOEt,-NO2 gave better yields,especially for 1-iodo-4- nirobenzene. Calix[4]arene derivatives 2 (2a-2c)with different fluorescent properties due to the difference of the electron donating and electron-withdrawing substituents. Calixarenes contains electron-withdrawing groups,such as COOC2H5 and NO2,which are not apparently fluorescent in aprotic solvents, while the electron-donating groups such as OCH3 may enhance fluorescence intensity. This effect was estimated mainly due to the enhancement of the p-π electron delocalization.
In order to study the recognition properties for phenols,the fluorescence experiments were carried out using 10 equiv. of calix[4]arene-2c. As shown in Fig. 1(a),p-nitrophenol and onitrophenol can significantly quench the fluorescence of calix[4]- arene-2c,but others had little effect on fluorescence. Further fluorescence titration experiments are shown in the Fig. 1(b). The fluorescence was monitored by the addition of increased concentrations of p-nitrophenol to the calix[4]arene-2c (5 × 10-6 mol/L). It was found that while no shift in the fluorescence maximum was observed,the fluorescence intensity of 2c gradually decreased with the addition of increased concentrations of d1. The association constant (Ka) of 2c for d1 was calculated to be 3.35 × 104 L/mol using the Benesie Hilderbrand equation. Meanwhile,the association constant (Ka) of 2c for d2 was calculated to be 2.53 × 104 L/mol by the fluorescence titration experiment. Compared to the Ka (2c-d2),the Ka (2c-d2) is higher,which indicated a stronger binding of 2c for d1. A preliminary study illustrates that calix[4]arene-2c can differentiate nitro-phenols from other phenol derivatives through fluorescence quenching.

|
Download:
|
| Fig. 1.(a) Fluorescence intensity changes for calix[4]arene (5× 10-6mol/L) in CH3CN upon addition of d1-d8 (10 equiv. of host). (b) Fluorescence spectra titration of 2c(5× 10-6mol/L) with various equivalents of d1 in CH3CN (λex = 279 nm,slit = 5). (c) The structures of the guests. | |
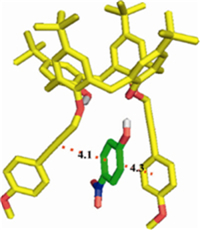
In order to understand the structural features of the complexes formed between the host and guests,computational calculations were carried out at HF/6-31G level using Gaussian 03 [14]. As Fig. 2 shows,the p-p stacking took place between the p-nitrophenol and calix[4]arene-2c,and formed a tweezer structure [15],which led to electron transfer and caused fluorescence quenching. The results from molecular mechanics calculations may provide more mechanistic insights.
|
Download:
|
| Fig. 2.The complex of cali[4]arene-2c with d1 by the theoretical calculation. | |
A novel series of calix[4]arene derivatives 2a-c were synthesized using the Sonogashira reaction in good yields. The calix[4]arene derivative 2c exhibited a high binding affinity and recognition properties toward nitro-phenols by the significant fluorescence quenching phenomenon. Computational calculations revealed a tweezer-like host-guest interacting mode. Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21372092,21072072, 21102051),PCSIRT (No. IRTO953),Program for New Century Excellent Talent in University (No. NCET-10-0428),self-determined research funds of CCNU from the colleges,basic research and operation of MOE (Nos. CCNU11C01002,CCNU13F005),and State Key Laboratory of Chemo/Biosensing and Chemometrics (201003). x;h
| [1] | I. Aoki, T. Sakaki, S. Shinka, A new metal sensory system based on intramolecular fluorescence quenching on the ionophoric calix[4]arene ring, J. Chem. Soc. Chem. Commun. (1992) 730-732. |
| [2] | T. Jin, K. Ichikawa, T. Koyama, A fluorescent calix[4]arene as an intramolecular excimer-forming Na+ sensor in nonaqueous solution, J. Chem. Soc. Chem. Commun. (1992) 499-501. |
| [3] | I. Leray, F. O'Reilly, J.L. Habib Jiwan, J.Ph. Soumillion, B. Valeur, A new calix[4]- arene-based fluorescent sensor for sodium ion, Chem. Commun. (1999) 795-796. |
| [4] | R. Joseph, C.P. Rao, Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors, Chem. Rev. 111 (2011) 4658-4702. |
| [5] | H. Chen, Z.L. Zou, S.L. Tan, et al., Efficient synthesis of water-soluble calix[4]arenes via thiol-ene ""click"" chemistry, Chin. Chem. Lett. 24 (2013) 367-369. |
| [6] | H.Y. Guo, F.F. Yang, Z.Y. Jiao, J.R. Lin, Click synthesis and dye extraction properties of novel thiacalix[4]arene derivatives with triazolyl and hydrogen bonding groups, Chin. Chem. Lett. 24 (2013) 450-452. |
| [7] | H. Al-Saraierh, D.O. Miller, P.E. Georghiou, Narrow-rim functionalization of calix[4]arenes via Sonogashira coupling reactions, J. Org. Chem. 70 (2005) 8273-8280. |
| [8] | G. Hennrich, M.T. Murillo, P. Prados, et al., Tetraalkynyl calix[4]arenes with advanced NLO properties, Chem. Commun. 21 (2005) 2747-2749. |
| [9] | M.H. Lee, D.T. Quang, H.S. Jung, et al., Ion-induced FRET on-off in fluorescent calix[4]arenes, J. Org. Chem. 72 (2007) 4242-4245. |
| [10] | M. Kumar, J.N. Babu, V. Bhalla, Fluorescent chemosensor for Cu2+ ion based on iminoanthryl appended calix[4]arene, J. Incl. Phenom. Macrocycl. Chem. 66 (2010) 139-145. |
| [11] | S.H. Kim, J.K. Choi, S.K. Kim, et al., On/off fluorescence switch of a calix[4]arene by metal ion exchange, Tetrahedron Lett. 47 (2006) 3737-3741. |
| [12] | Z. Asfari, A. Bilyk, C. Bond, et al., Factors influencing solvent adduct formation by calixarenes in the solid state, Org. Biomol. Chem. 2 (2004) 387-396. |
| [13] | M.J. Chetcuti, A.M.J. Devoille, A. Ben Othman, et al., Synthesis of mono-, di- and tetra-alkyne functionalized calix[4]arenes: reactions of these multipodal ligands with dicobalt octacarbonyl to give complexes which contain up to eight cobalt atoms, Dalton Trans. 16 (2009) 2999-3008. |
| [14] | M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford, CT, 2004. |
| [15] | H.D. Matthias, S. Hannes, Z. Matthias, A.A. Vladimir, Rationally designed calix[4]- arene-pyrrolotetrathiafulvalene receptors for electron-deficient neutral guests, J. Org. Chem. 78 (2013) 4905-4912. |




