b College of Chemical and Pharmaceutical Engineering, Henan University of Science and Technology, Luoyang 470003, China
As well known,chelating material is a kind of materials with functional groups containing one or more (multiple) atoms of oxygen,nitrogen,sulfur,phosphorus,which can be covalently bonded to metal ions. Commercially available chelating/ion exchange materials with amino or iminodiacetic acid groups are widely used to remove heavy metal from aqueous solution [1, 2, 3]. Compared to granular materials,fiber is known to have large apparent specific surface,excellent osmotic stability and good kinetic properties. As a result of these properties,the intensive research has been carried out to develop the preparation methods for different types of fibrous chelating materials.
Many methods are used for prepare chelating fibers,such as chelating fibers with chemical grafting [4, 5],radiation-inducing, electron-beam-induced grafting [6, 7],and chemical modifications [8, 9]. However,these preparation techniques are cumbersome and ineffective because two or more steps are required for the preparation of chelating fibers,and demand 60Coγ or high-energy electron irradiation for the grafting processes. Polyphenylene sulfide (PPS) fiber is a new type performance material with excellent mechanical strength,chemical and thermal stability. Due to its rich benzene rings,our laboratory has prepared strong acid/ base ion exchange fibers on PPS fiber [10, 11] with high exchange capacity and excellent chemical stability. The advantage of PPS fiber-based preparations is that the irradiation by 60Coγ or highenergy electron was avoided.
In the present work,we prepared a series of chelating functional fibers through chloromethylation and further functionalization on the PPS fiber. The structure and properties were proven by FT-IR, elementary analysis,thermogravimetric analysis and SEM-EDS. 2. Experimental
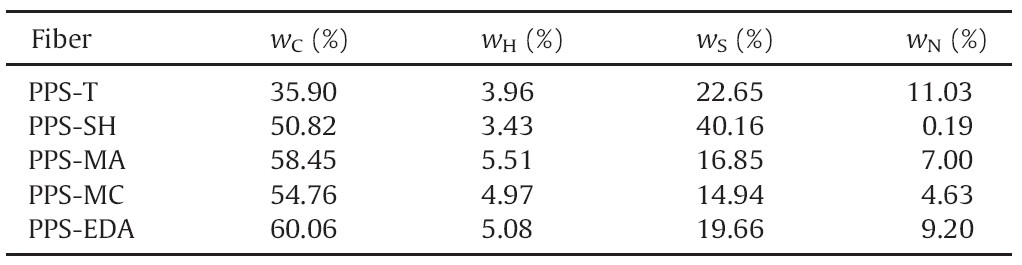
Chloromethylated polyphenylene sulfide (CMPPS) fiber was prepared according to the method previously reported [12]. As shown in Scheme 1,the chelating fiber was prepared.
|
Download:
|
| Scheme 1.Schematic illustration of the preparation of PPS chelating fiber. | |
About 1 g of CMPPS fiber (C,H,S and Cl content were 52.05%, 3.09%,20.07% and 24.79%,respectively) was swelled in 1,4-dioane for 12 h. And then 30 mL of ethanol,2 g of thiourea and a phase transfer catalyst tetrabutyl ammonium bromide (TBAB,0.02 g) were added. The mixture was heated in an oil bath at 60℃ for 12 h. The fiber was filtered and rinsed thoroughly with distilled water to remove residual impurities. The products were left to dry completely in vacuum to produce the thiourea chelating fiber PPS-T (C,35.90%; H,3.96%; S,22.65%; N,11.03%).
The PPS-T fiber was subjected to a hydrolysis reaction with 40 mL,20% aq. NaOH at 80℃ for 12 h. The product was washed with distilled water till filtrate was neutral,then left to dry completely in vacuum for 24 h to obtain the thiol chelating fiber PPS-SH (C,50.82%; H,3.43%; S,40.16%; N,0.19%). 2.2. Preparation of the amino and acylamide chelating fibers
About 1 g of CMPPS (weight gain was 38.69% and C,H,S and Cl content were 52.16%,3.24%,21.60% and 23.00%,respectively) was immersed in 40 mL of 25% aqueous methylamine for 8 h,and then the reaction was carried out at 40℃ for 24 h. The fiber was filtered and soaked in 1 mol/L NaOH for at least 8 h. The product was rinsed thoroughly to neutral pH with distilled water and dried at 80℃ under vacuum to a constant weigh to give PPS-MA (C,58.45%; H, 5.51%; S,16.85%; N,7.00%).
The above fiber was put into a flask containing 25 mL of acetic anhydride and the reaction mixture was heated in an oil bath for 8 h. The resulting product was filtered and rinsed thoroughly with distilled water until the filtrate was neutral,then left to dry completely in vacuum for several days to produce PPS-MC (C, 54.76%; H,4.97%; S,14.94%; N,4.63%). 2.3. Preparation of the ethylenediamine chelating fiber
About 1 g of CMPPS fiber (C,H,S and Cl contents were 52.16%, 3.24%,21.60% and 23.00%,respectively) was swelled in 1,2- dichloroethane for 12 h,and then 25 mL of ethylenediamine was added quickly,the reaction mixture was heated in an oil bath at 90℃ for 24 h. After the reaction was complete,the fiber was filtered and soaked in 1 mol/L NaOH for at least 8 h,and then fiber was washed to neutral pH with distilled water and dried at 80℃ under vacuum to constant weigh to give PPS-EDA (C,60.06%; H, 5.08%; S,19.66%; N,9.20%). 2.4. Characterization of the chelating fiber
The chelating fibers were characterized by Fourier transform infrared spectroscopy (FT-IR 20009 AmericanThermo Nicolet Co., Ltd.),elementary analysis (EA 1112 Thermo Flash,USA),scanning electron microscopy microscope-energy X-ray spectroscopy (JSM- 7500F JEOL,Japan),and thermogravimetric and differential thermogravimetric analysis (STA 409 PC NETZSCH,Germany). 3. Results and discussion
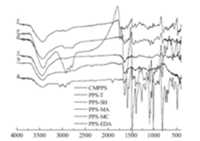
To identify reactions,FT-IR spectra of the PPS chelating fiber were analyzed. Fig. 1 showed the spectra of chelating PPS fiber. The characteristic bands at 1259 cm-1 (CH2Cl) and 705 cm-1(C-Cl) were found (curve a) [13]. In Fig. 1,curve b,the new adsorption peaks of stretching vibration at 1644 cm-1 and 1109 cm-1 were attributed to C=N and C-S bonds in the thiourea group [14]. Furthermore,the stretching vibration adsorption band of -NH2 group appears at 3400 cm-1. The above changes indicated the formation of the thiourea group. As shown in curve c,the 1644 cm-1 vibration peak weakened but did not disappear completely,suggesting that some thiourea groups did not undergo the hydrolysis reaction. And the bands at 2540 cm-1 and 853 cm-1 (-SH) indicated the existence of the -SH group [15].
|
Download:
|
| Fig. 1.FT-IR spectra of chelating fibers: (a) CMPPS; (b) PPS-T; (c) PPS-SH; (d) PPSMA;(e) PPS-MC; (f) PPS-EDA. | |
The most important peaks around 1460 cm-1 in Fig. 1 (curves d-f) were attributed to the presence of aliphatic C-N bonds [16]. The broad absorption bands at 3400 cm-1 correspond to the stretching vibration of N-H bands. In curve d,a weak stretching vibration peak of benzylalcohol (C-OH) was situated at 1020 cm-1, indicating a partial hydrolysis of the benzyl chloride. In curve e, two new adsorption peaks at 1742 cm-1 and 1695 cm-1 can be ascribed to the C=O stretching vibrations of ester and tertacylamide group,respectively [17]. It needs to be pointed out that all of above FT-IR spectra of chelating fibers,the 1259 cm-1 and 705 cm-1 vibration peaks weakened but did not disappear completely,demonstrating the incomplete reaction of the chloromethyl group. The results of FT-IR suggested that chelating fiber had been made successfully and effectively. These results are consistent with the elementary analysis.
The results of element analysis of the chelating fibers were shown in Table 1. The weight gain of PPS-T fiber was 36.42% after the thiourea reaction,according to elementary analysis of PPS-T (C, 35.90%; H,3.96%; S,22.65%; N,11.03%) and the content of thiourea was calculated to be 3.94 mmol/g. In the PPS-SH fiber (C,50.82%; H, 3.43%; S,40.16%; N,0.19%),the C and S content increased clearly, and the N percentage decreased obviously compared with that of PPS-T fiber,and the thiol content in the fiber calculated from the element analysis was 3.85 mmol/g. This result suggested that most of thiourea groups were hydrolyzed.
| Table 1 Elemental analysis of chelating fibers. |
The content of the nitrogen of the PPS-MA and PPS-EDA fiber obtained by elemental analysis was 7.00% and 9.20%,respectively. The amine group content in the fiber calculated from the element analysis was 5.00 and 6.57 mmol/g,and the exchange capacity of the PPS-MA fiber was 5.08 mmol/L and 6.52 mmol/g by acid-base titration,respectively. According to the element analysis we infer that CMPPS fiber underwent a partial hydrolysis reaction under alkaline aqueous solution condition because the high reactivity of benzyl chloride. In PPS-MC the N value decreased clearly compared with that of PPS-MA fiber,suggesting that the acylamide groups were successfully anchored on the fiber. In addition,the ester groups were also anchored on the PPS-MC fiber because the existence of hydroxyl groups on PPS-MA. Therefore we have obtained the bifunctional adsorptive fibers containing amino/ hydroxyl or acylamide/ester.
Fig. 2a showed the weight loss and the rate of weight loss curve obtained during the pyrolysis of the chelating fibers under inert atmosphere at a heating rate of 10℃/min. It is observed that each chelating fiber exhibited a similar decomposition profile. From room temperature to 600℃,the total mass loss of PPS fiber was 42%,and its decomposition peak was found at 560℃. Generally, the decomposition profile depicted that first peak exists about 100℃ and it could be assigned to the dehydration of moisture within the fiber,but the peak height of PPS-SH,PPS-MA,and PPSMC fiber was quite low. The mass loss of PPS-T and PPS-EDA fiber was approximately 5%,fiber has high hygroscopicity because of the particularity of their functional groups.

|
Download:
|
| Fig. 2.Thermal stability of chelating fiber. | |
As can be seen from the TG and DTG curves of the chelating fibers,a decomposition step was observed from 230℃ to 420℃ with a major weight loss,suggesting the decomposition of the functional groups on fiber occurred. The decomposition peaks were also observed at 262℃ (PPS-T),325℃ (PPS-SH),373℃ (PPSMA), 378℃ (PPS-MC) and 325℃ (PPS-EDA),respectively. And approximately 45% weight loss was observed for our chelating fiber from room temperature to 600℃,but a 55% weight loss for PPS-T fiber was observed. Ma [18] reported that an amine groupcontaining chelating material based on polypropylene fiber with a decomposition temperature of 210℃ and the total mass loss of more than 80% during the pyrolysis process. The result indicates that our chelating fiber has excellent thermal stability.
In order to evaluate the surface morphologies of the chelating fibers,their SEM images were taken as shown in Fig. 3. The surface morphology of the chelating fiber was rough in comparison with the smooth surface of the CMPPS fiber and the change in surface of the chelating fiber was probably influenced by the group anchored on the fiber. Another reason is that the crystallinity of the chelating fiber was damaged through functional reaction. The energy dispersive spectrometer (EDS) analysis indicated that the element has slight changed from surface to the center along cross section of chelating fiber. It is observed that the Cl content was 24.72% in CMPPS fiber,and decreased clearly in chelating fiber. However,the N content also changed in the chelating fiber. The result is consistent with the element analysis.

|
Download:
|
| Fig. 3.SEM images and EDS of chelating fibers: (a) CMPPS; (b) PPS-T; (c) PPS-SH; (d) PPS-MA; (e) PPS-MC; (f) PPS-EDA. | |
A series of chelating fibers has been successfully prepared from polyphenylene sulfide fiber. The thiourea content was 3.94 mmol/ g,thiol content was 3.85 mmol/g,and the exchange capacity of PPS-MA and PPS-EDA was 5.00 mmol/g and 6.57 mmol/g,respectively. Thermogravimetric analysis confirmed that the chelating fiber exhibited excellent thermal stability. Acknowledgments
We gratefully acknowledge generous support by the National Natural Science Foundation of China (No. 20574063),Doctoral Foundation of Ministry of Education of China (No.20104101110005). dU
| [1] | Y. Sun, Z.C. Li, Y. Xu, Preparation and application of a novel orotic acid chelating resin for removal of Cu(Ⅱ) in aqueous solutions, Chin. Chem. Lett. 24 (2013) 747- 750. |
| [2] | S. Kagaya, E. Maeba, Y. Inoue, et al., A solid phase extraction using a chelate resin immobilizing carboxymethylated pentaethylenehexamine for separation and preconcentration of trace elements in water samples, Talanta 79 (2009) 146-152. |
| [3] | S. Kagaya, H. Miyazaki, Y. Inoue, et al., Chelating fibers prepared with a wet spinning technique using a mixture of a viscose solution and a polymer ligand for the separation of metal ions in an aqueous solution, J. Hazard. Mater. 203-204 (2012) 370-373. |
| [4] | M. Monier, N. Nawar, D.A. Abdel-Latif, Preparation and characterization of chelating fibers based on natural wool for removal Hg(Ⅱ), Cu(Ⅱ) and Co(Ⅱ) metal ions from aqueous solution, J. Hazard. Mater. 184 (2010) 118-125. |
| [5] | Y. Tian, M. Wu, R.G. Liu, et al., Modified native cellulose fibers - a novel efficient adsorbent for both fluoride and arsenic, J. Hazard. Mater. 185 (2011) 93-100. |
| [6] | L. Xu, J.N. Wang, Y. Meng, A.M. Li, Fast removal of heavy metal ions and phytic acids from water using new modified chelating fiber, Chin. Chem. Lett. 23 (2012) 105-108. |
| [7] | A. Jyo, J.Y. Hamabe, H. Matsuura, et al., Preparation of bifunctional chelating fiber containing iminodi(methylphosphonate) and sulfonate and its performances in column-mode uptake of Cu(Ⅱ) and Zn(Ⅱ), React. Funct. Polym. 70 (2010) 508-515. |
| [8] | K. Ikeda, D. Umeno, K. Sario, et al., Removal of boron using nylon-based chelating fibers, Ind. Eng. Chem. Res. 50 (2011) 5727-5732. |
| [9] | L.H. Zhang, X.S. Zhang, P.P. Li, W.Q. Zhang, Effective Cd2+ chelating fiber based on polyacrylonitrile, React. Funct. Polym. 69 (2009) 48-54. |
| [10] | Y. Meng, J.N. Wang, L. Xu, A.M. Li, Fast removal of Pb2+ from water using new chelating fiber modified with acylamino and amino groups, Chin. Chem. Lett. 23 (2012) 496-499. |
| [11] | X.R. Li, J.J. Huang, S.G. Yuan, Preparation of polyphenylene sulfide strong acid ion exchange fiber and the adsorption properties for Cr(Ⅲ), Polym. Mater. Sci. Eng. 28 (2012) 145-149. |
| [12] | J.J. Huang, X. Zhang, L.L. Bai, S.G. Yuan, Polyphenylene sulfide based anion exchange fiber: synthesis, characterization and adsorption of Cr(VI), J. Environ. Sci. 24 (2012) 1433-1438. |
| [13] | L.Q. Yang, Y.F. Li, X.L. Jin, et al., Synthesis and characterization of a series of chelating resins containing amino/imino-carboxyl groups and their adsorption behavior for lead in aqueous phase, Chem. Eng. J. 168 (2011) 115-124. |
| [14] | Q.S. Ren, W.Q. Huang, B.L. He, Synthesis of polystyrylsulfonylthiourea resin and its chelate property for Au3+ ion, Ion Exch. Adsorp. 5 (1989) 131-134. |
| [15] | X.M. Wu, C.H. Xiong, Z.N. Shu, Adsorption of silver onto thiol-resin and its mechanism, J. Chem. Ind. Eng. 54 (2003) 1466-1469. |
| [16] | A. Wolowicz, Z. Hubicki, The use of the chelating resin of a new generation Lewatit Monoplus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions, Chem. Eng. J. 197 (2012) 493-508. |
| [17] | L. Niu, S.B. Deng, G. Yu, J. Huang, Efficient removal of Cu(Ⅱ), Pb(Ⅱ), Cr(VI) and As(V) from aqueous solution using an aminated resin prepared by surface-initiated atom transfer radical polymerization, Chem. Eng. J. 165 (2010) 751-757. |
| [18] | N.F. Ma, Y. Yang, S.X. Chen, Q.K. Zhang, Preparation of amine group-containing chelating fiber for thorough removal of mercury ions, J. Hazard. Mater. 171 (2009) 288-293. |