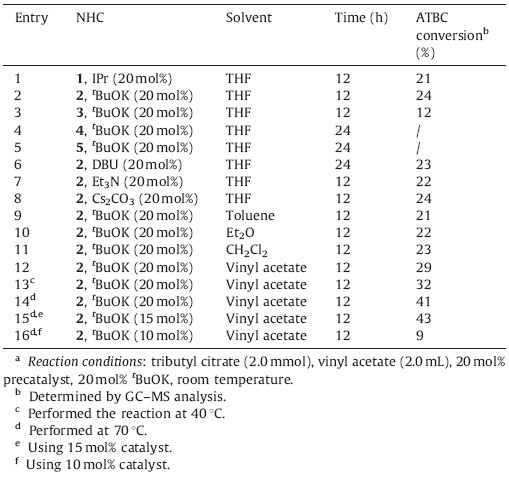
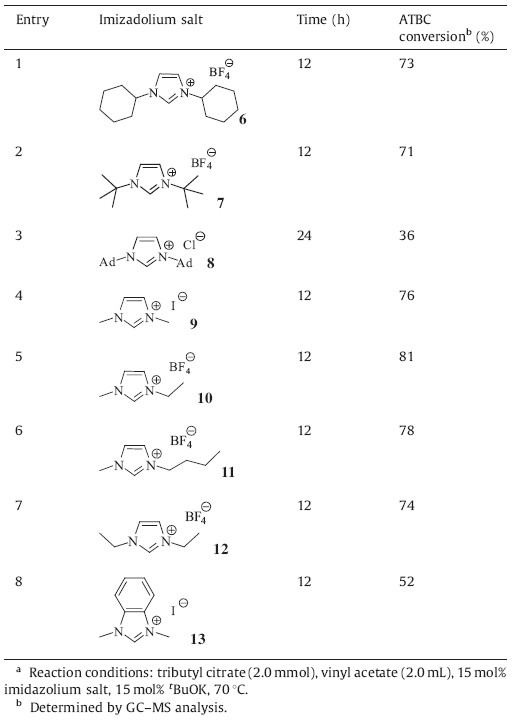
N-Heterocyclic carbenes (NHCs) have attracted considerable attention in the past decade,which have been utilized successfully in both transition-metal-catalyzed reactions [1] and organocatalyzed reactions [2]. As versatile nucleophilic organocatalysts,NHCs can catalyze a variety of important transformations such as benzoin reaction [3],Stetter reaction [4],homoenolate reactions [5] via polarity reversal of carbonyl compounds. We and others have also demonstrated that NHCs can be used as the catalyst for redox reactions [6],the cyanation reaction [7],the aldol reaction [8] and several other reactions [9]. Aside from these applications, NHCs have been used as highly efficient organocatalysts for acyl transfer reactions. More than ten years ago,the group of Nolan [10] and Hedrick [11] reported the first two examples of NHC-catalyzed transesterification reaction independently. Following these seminal findings,great effort has been devoted to the NHC-promoted transesterifications,including their application in polymerization reactions [12].
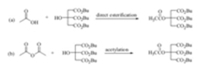
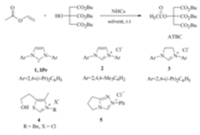
Acetyltributylcitrate (ATBC) is an ester derived from citric acid, which can be used to replace phthalate esters as a green and nontoxic plasticizer in many plastic products such as toys,child care articles,food packages,medicinal instruments and so on. To date,this environmentally friendly plasticizer is mainly prepared by two methods (Scheme 1),namely,the direct esterification of tributyl citrate with HOAc [13] and the acetylation of tributyl citrate with Ac2O [14]. In line with our continuing interest in NHCs catalysis [15],we found that NHCs can catalyze the transesterification reaction between vinyl acetate and tributyl citrate to afford ATBC in high yield (Scheme 2),which might be used as the third method for the preparation of this green plasticizer. Herein, we would like to disclose our preliminary results.
|
Download:
|
| Scheme 1.The synthetic method of ATBC. | |
|
Download:
|
| Scheme 2.NHCs-catalyzed synthesis of ATBC. | |
To a solution of 10 (60 mg,0.3 mmol) in 0.5 mL THF was added tBuOK (34 mg,0.3 mmol) under N2. After the mixture was stirred for 30 min at rt,tributyl citrate (720 mg,2.0 mmol) was added followed by vinyl acetate (1.0 mL). The reaction mixture was then stirred at 70℃ for 12 h. The solution was filtered through a short silica pad and concentrated. The crude products were purified using silica-gel chromatography (ethyl acetate/petroleumether) to afford ATBC as a colorless oil. Yield: 81%; Rf = 0.25 (petroleum ether-EtOAc,15:1); IR (KBr,cm-1): 3498,2962,2874,1740,1466, 1188; 1H NMR (400 MHz,CDCl3): δ 4.21 (t,2H,J = 6.7 Hz),4.09 (m, 4H,J = 6.7 Hz),2.84 (q,4H,J = 38.8,15.5 Hz),2.05 (s,3H),1.71-1.55 (m,6H),1.45-1.30 (m,6H),0.97-0.88 (m,9H); GC-MS (EI): m/z 402 (M+). 3. Results and discussion
Our investigation commenced with the transesterification between vinyl acetate and tributyl citrate in the presence of a catalytic amount of 1,3-bis(2,6-diisopropylphenyl)imidazol-2- ylidene (IPr,a stable NHC) [16]. To our delight,under the catalysis of 20 mol% IPr,the reaction proceeded smoothly in THF at room temperature,giving 21% conversion of ATBC (Table 1,entry 1). Encouraged by this result,a range of simple NHC precursors such as imidazolium 2,imidazolinium 3,thiazolium 4 and triazolium 5 were tested for the reaction. The results indicated that NHCs generated from 2 or 3 can promote the reaction with low efficiency (Table 1,entries 2 and 3),whereas NHCs generated from 4 and 5 cannot catalyze the transesterification (Table 1,entries 4 and 5). tKOBu,DBU,Et3N and Cs2CO3 all can react with the azolium salts to produce the corresponding NHC catalyst in situ,but no obvious differences were observed (Table 1,entries 6-8). Other reaction media,such as toluene,diethyl ether,DCM were also surveyed briefly and the results showed that vinyl acetate was the best choice with respect to the conversion of ATBC (Table 1,entry 9-12). When the reaction temperature was elevated to 40℃ and 70℃, the conversion of ATBC can be increased to 32% and 41% respectively (Table 1,entries 13 and 14). Lowering the catalyst loading to 15 mol% had no obvious effects on the reaction (Table 1, entry 15). However,further reduction of NHC to 10 mol% led to a dramatic decrease of the conversion of ATBC (Table 1,entry 16). Therefore,the optimized conditions were determined as follows: 15 mol% imidazolium salt as precatalyst and 15 mol% tKOBu as base to generate NHC in situ in vinyl acetate at 70℃ for a given period of time.
| Table 1 Evaluation of conditions for transesterification reaction.a |
Since the steric and electronic properties of the N-substituents in NHCs had an obvious effect on their nucleophilicity,a series of imidazolium salts with different N-substituents were then investigated for the transesterification reaction under the optimal reaction conditions as mentioned above. Using more nucleophilic alkyl-substituted NHC 6 or 7 as catalyst precursors instead of IMes·HCl,the catalytic efficiency of NHC increased significantly, giving >70% conversion of ATBC (Table 2,entries 1 and 2). The stable NHC IAd,owing to the large bulky adamantyl group,only catalyzed the reaction with moderate efficiency with 36% conversion of ATBC (Table 2,entry 3). Several alkyl-substituted ionic liquids such as 9,10,11 and 12 were also evaluated for the reaction (Table 2,entries 4-7). In the presence of tKOBu,the ionic liquid converted to the corresponding NHC in situ,which can promote the transesterification efficiently. Benzimidazolium salt 13 was also tested for the reaction as a precatalyst,which can give 52% conversion of the desired product (Table 2,entry 8).
| Table 2 NHC-Catalyzed transesterification reaction of vinyl acetate with tributyl citrate.a |
In conclusion,we have developed a NHC-promoted transesterification reaction between tributyl citrate and vinyl acetate. The mild conditions and simple procedures provide a new approach for the synthesis of ATBC. Further studies on the scale-up and applications of this methodology are ongoing in our laboratory. Acknowledgments
We are gratefully acknowledge the financial support of the Doctor Foundation of Bingtuan (No. 2011BB011) and Shihezi University.
| [1] | S. Díez-González, N. Marion, S.P. Nolan, N-heterocyclic carbenes in late transition metal catalysis, Chem. Rev. 109 (2009) 3612-3676. |
| [2] | (a) D. Enders, O. Niemeier, A. Henseler, Organocatalysis by N-heterocyclic carbenes, Chem. Rev. 107 (2007) 5606-5655; (b) N. Marion, S. Díez-González, S.P. Nolan, N-heterocyclic carbenes as organocatalysts, Angew. Chem. Int. Ed. 46 (2007) 2988-3000; (c) V. Nair, S. Vellalath, B.P. Babu, Recent advances in carbon-carbon bondforming reactions involving homoenolates generated by NHC catalysis, Chem. Soc. Rev. 37 (2008) 2691-2698; (d) D. Enders, A. Grossmann, N-heterocyclic carbene catalyzed domino reactions, Angew. Chem. Int. Ed. 51 (2012) 314-325. |
| [3] | (a) D. Enders, A. Grossmann, J. Fronert, G. Raabe, N-heterocyclic carbene catalysed asymmetric cross-benzoin reactions of heteroaromatic aldehydes with trifluoromethyl ketones, Chem. Commun. 46 (2010) 6282-6284; (b) D. Enders, A. Henseler, A direct intermolecular cross-benzoin type reaction: N-heterocyclic carbene-catalyzed coupling of aromatic aldehydes with trifluoromethyl ketones, Adv. Synth. Catal. 351 (2009) 1749-1752. |
| [4] | (a) T. Jousseaume, N.E. Wurz, F. Glorius, Highly enantioselective synthesis of aamino acid derivatives by an NHC-catalyzed intermolecular Stetter reaction, Angew. Chem. Int. Ed. 50 (2011) 1410-1414; (b) X.Q. Fang, X.K. Chen, H. Lv, Y.R. Chi, Enantioselective Stetter reactions of enals and modified chalcones catalyzed by N-heterocyclic carbenes, Angew. Chem. Int. Ed. 50 (2011) 11782-11785; (c) D.A. DiRocco, T. Rovis, Catalytic asymmetric intermolecular Stetter reaction of enals with nitroalkenes: enhancement of catalytic efficiency through bifunctional additives, J. Am. Chem. Soc. 133 (2011) 10402-10405. |
| [5] | (a) X.K. Chen, X.Q. Fang, Y.R. Chi, cis-Enals in N-heterocyclic carbene-catalyzed reactions: distinct stereoselectivity and reactivity, Chem. Sci. 4 (2013) 2613- 2618; (b) D.T. Cohen, K.A. Scheidt, Cooperative Lewis acid/N-heterocyclic carbene catalysis, Chem. Sci. 3 (2012) 53-57; (c) J.M. Mo, X.K. Chen, Y.R. Chi, Oxidative, g-Addition of enals to trifluoromethyl ketones: enantioselectivity control via Lewis acid/N-heterocyclic carbene cooperative catalysis, J. Am. Chem. Soc. 134 (2012) 8810-8813. |
| [6] | (a) D.A. DiRocco, T. Rovis, Catalytic asymmetric 伪-acylation of tertiary amines mediated by a dual catalysis mode: N-heterocyclic carbene and photoredox catalysis, J. Am. Chem. Soc. 134 (2012) 8094-8097; (b) Y.M. Zhao, Y. Tam, Y.J. Wang, Z. Li, J. Sun, N-heterocyclic carbene-catalyzed internal redox reaction of alkynals: an efficient synthesis of allenoates, Org. Lett. 14 (2012) 1398-1401; (c) F.G. Sun, L.H. Sun, S. Ye, N-heterocyclic carbene-catalyzed enantioselective annulation of bromoenal and 1,3-dicarbonyl compounds, Adv. Synth. Catal. 353 (2011) 3134-3138; (d) X.B. Wang, X.L. Zou, G.F. Du, Z.Y. Liu, B. Dai, Nucleophilic carbene-catalyzed redox-esterification reaction of a-halo-a,b-unsaturated aldehyde, Tetrahedron 68 (2012) 6498-6503. |
| [7] | (a) J.J. Song, F. Gallou, J.T. Reeves, et al., Activation of TMSCN by N-heterocyclic carbenes for facile cyanosilylation of carbonyl compounds, J. Org. Chem. 71 (2006) 1273-1276; (b) Y. Suzuki, A. Bakar, K. Muramatsu, M. Sato, Cyanosilylation of aldehydes catalyzed by N-heterocyclic carbenes, Tetrahedron 62 (2006) 4227-4231; (c) J. Zhang, G.F. Du, Y.K. Xu, L. He, B. Dai, N-heterocyclic carbene catalyzed cyanation reaction of carbonyl compounds with ethyl cyanoformate and acetyl cyanide, Tetrahedron Lett. 52 (2011) 7153-7156. |
| [8] | (a) J.J. Song, Z.L. Tan, J.T. Reeves, N.K. Yee, C.H. Senanayake, N-heterocyclic carbene-catalyzed Mukaiyama aldol reactions, Org. Lett. 9 (2007) 1013-1016; (b) G.F. Du, L. He, C.Z. Gu, B. Dai, N-heterocyclic carbene catalyzed vinylogous aldol reaction of 2-(trimethylsilyloxy) furan and aldehydes, Synlett (2010) 2513- 2517. |
| [9] | (a) T.Y. Jian, L. He, C. Tang, S. Ye, N-heterocyclic carbene catalysis: enantioselective formal [2+2] cycloaddition of ketenes and N-sulfinylanilines, Angew. Chem. Int. Ed. 50 (2011) 9104-9107; (b) X.D. Zhao, K.E. Ruhl, T. Rovis, N-heterocyclic-carbene-catalyzed asymmetric oxidative hetero-Diels-Alder reactions with simple aliphatic aldehydes, Angew. Chem. Int. Ed. 51 (2012) 12330-12333; (c) J. Dugal-Tessier, E.A. O'Bryan, T.B.H. Schroeder, D.T. Cohen, K.A. Scheidt, An Nheterocyclic carbene/Lewis acid strategy for the stereoselective synthesis of spirooxindole lactones, Angew. Chem. Int. Ed. 51 (2012) 4963-4967; (d) L. Candish, D.W. Lupton, N-heterocyclic carbene-catalyzed Ireland-coates Claisen rearrangement: synthesis of functionalized b-lactones, J. Am. Chem. Soc. 135 (2013) 58-61. |
| [10] | G.A. Grasa, R.M. Kissling, S.P. Nolan, N-heterocyclic carbenes as versatile nucleophilic catalysts for transesterification/acylation reactions, Org. Lett. 4 (2002) 3583-3586. |
| [11] | G.W. Nyce, J.A. Lamboy, E.F. Connor, R.M. Waymouth, J.L. Hedrick, Expanding the catalytic activity of nucleophilic N-heterocyclic carbenes for transesterification reactions, Org. Lett. 4 (2002) 3587-3590. |
| [12] | (a) G.A. Grasa, R. Singh, S.P. Nolan, Transesterification/acylation reactions catalyzed by molecular catalysts, Synthesis (2004) 971-985; (b) M. Fèvre, J. Pinaud, Y. Gnanou, J. Vignolle, D. Taton, N-heterocyclic carbenes (NHCs) as organocatalysts and structural components in metal-free polymer synthesis, Chem. Soc. Rev. 42 (2013) 2142-2172. |
| [13] | A. Sakakura, S. Nakagawa, K. Ishihara, Bulky diarylammonium arenesulfonates as mild and extremely active dehydrative ester condensation catalysts, Tetrahedron 62 (2006) 422-433. |
| [14] | A. Sakakura, K. Kawajiri, T. Ohkubo, Y. Kosugi, K. Ishihara, Widely useful DMAPcatalyzed esterification under auxiliary base- and solvent-free conditions, J. Am. Chem. Soc. 129 (2007) 14775-14779. |
| [15] | (a) Z.H. Cai, G.F. Du, L. He, C.Z. Gu, B. Dai, N-heterocyclic carbene catalyzed hydrophosphonylation of aldehydes, Synthesis (2011) 2073-2078; (b) Y.C. Fan, G.F. Du, W.F. Sun, W. Kang, L. He, N-heterocyclic carbene-catalyzed cyanomethylation of aldehydes with TMSAN, Tetrahedron Lett. 53 (2012) 2231- 2233; (c) X.L. Zou, G.F. Du, W.F. Sun, et al., N-heterocyclic carbene mediated Reformatsky reaction of aldehydes with a-trimethylsilylcarbonyl compounds, Tetrahedron 69 (2013) 607-612. |
| [16] | A.J. Arduengo Ⅲ R. Krafczyk, R. Schmutzler, Imidazolylidenes, imidazolinylidenes and imidazolidines, Tetrahedron 55 (1999) 14523-14534. |