Halogenated aromatic amines are important intermediates for the synthesis of many fine chemicals,such as organic dyes, perfumes,herbicides,pesticides,preservatives,plant growth regulators,medicines and light sensitive or nonlinear optical materials [1, 2, 3]. They are generally produced by the catalytic hydrogenation of aromatic halogenated nitro compounds over Raney-Ni or supported noble metal catalysts in organic solvents [4, 5, 6, 7, 8, 9, 10]. These organic solvents used in these reactions are usually methanol or other alcohols. However,their usage has been considered not environmental friendly due to their toxicity,waste generation,flammability especially in the presence of ignitable metal catalysts,and high energy consumption for the recovery. Catalytic reactions under solvent-free or highly concentrated conditions have attracted much attention in recent years because of their prevention of waste generation,reduction in solvent costs and energy consumption,decrease in reaction times and/or batch size [11, 12]. Li et al. systematically investigated the hydrogenation of a series of halogenated nitrobenzenes over different noble metal catalysts supported on activated carbon [13, 14, 15]. Recently,Wang et al. investigated solvent-free selective hydrogenation of chloronitrobenzene to chloroaniline over a Pt/Fe3O4 catalyst [16].
In hydrogenation of o-chloronitrobenzene in ethanol,water addition improves the activity [17],the selectivity [1, 18] and the turnover frequency [19] of the catalysts. The increased catalytic properties upon water addition are mainly attributed to the competitive adsorption between water,solvent (ethanol) and reactants on surface active sites and the formation of hydrogen bonds between o-chloronitrobenze and water in the solvent In hydrogenation of o-chloronitrobenzene in ethanol,water addition improves the activity [17],the selectivity [1, 18] and the turnover frequency [19] of the catalysts. The increased catalytic properties upon water addition are mainly attributed to the competitive adsorption between water,solvent (ethanol) and reactants on surface active sites and the formation of hydrogen bonds between o-chloronitrobenze and water in the solvent [17, 18, 19]. However,in these studies,the effect of water on the hydrogenation of halogenated nitrobenzenes was carried out in ethanol in the presence of a large amount of water compared to the solvent. To the best of our knowledge,the effect of water,especially minor amount of water,on the hydrogenation of nitroaromatics under solvent-free conditions has not been reported. In this study,we investigated the role of minor amount of water in palladium catalyzed hydrogenation of halogenated nitrobenzenes under solvent-free conditions. First,we found that dried sponge Pd has no catalytic activity for the hydrogenation of halogenated nitrobenzenes under solvent-free conditions. After the addition of minor amount of water,the catalytic properties of Pd/C increased significantly. The relationship between the minor amount of water added and the selectivity of hydrogenation was studied to evaluate the optimum amount of water for the reaction.[17, 18, 19]. However,in these studies,the effect of water on the hydrogenation of halogenated nitrobenzenes was carried out in ethanol in the presence of a large amount of water compared to the solvent. To the best of our knowledge,the effect of water,especially minor amount of water,on the hydrogenation of nitroaromatics under solvent-free conditions has not been reported.
In this study,we investigated the role of minor amount of water in palladium catalyzed hydrogenation of halogenated nitrobenzenes under solvent-free conditions. First,we found that dried sponge Pd has no catalytic activity for the hydrogenation of halogenated nitrobenzenes under solvent-free conditions. After the addition of minor amount of water,the catalytic properties of Pd/C increased significantly. The relationship between the minor amount of water added and the selectivity of hydrogenation was studied to evaluate the optimum amount of water for the reaction.
Pd/C catalysts with a nominal Pd loading of 2.0 wt% were prepared by the impregnation method. A detailed impregnation process had been given elsewhere [20]. 2 mL of H2PdCl4 aqueous solution (0.05 g/mL) was dropped into the suspension of 5 g of pretreated activated carbon,and the solution pH value of 10-11 was then reached by the addition of NaOH aqueous solution, eventually the catalyst Pd/C was reduced by hydrazine hydrate. The resulting catalyst was washed with distilled water until its pH value was about 7,and degassed in vacuum at 383 K for 10 h for five times,then kept in a vacuum drying oven. The solvent-free hydrogenation of 6-chloro-2-nitrotoluene (6-CNT) was carried out in a 75 mL stainless steel autoclave at the H2 pressure of 1 MPa with a stirring rate of 1200 rpm. For comparison,the hydrogenation of aromatic halogenated nitro compounds with the addition of water was carried out in the same autoclave under the identical reaction conditions.
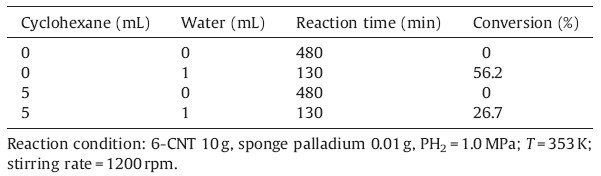
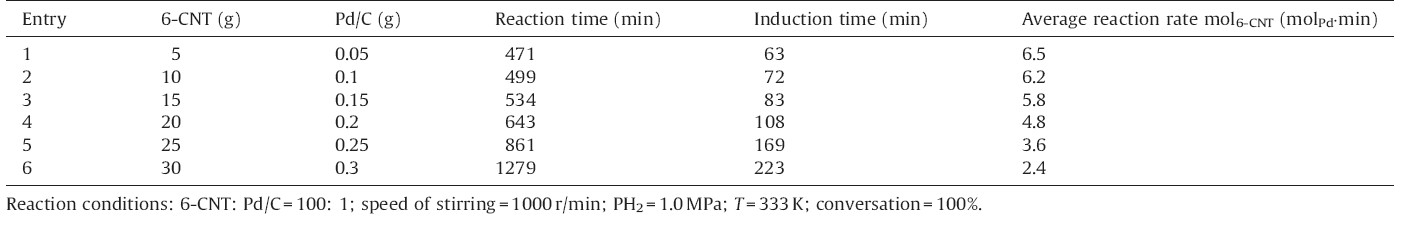
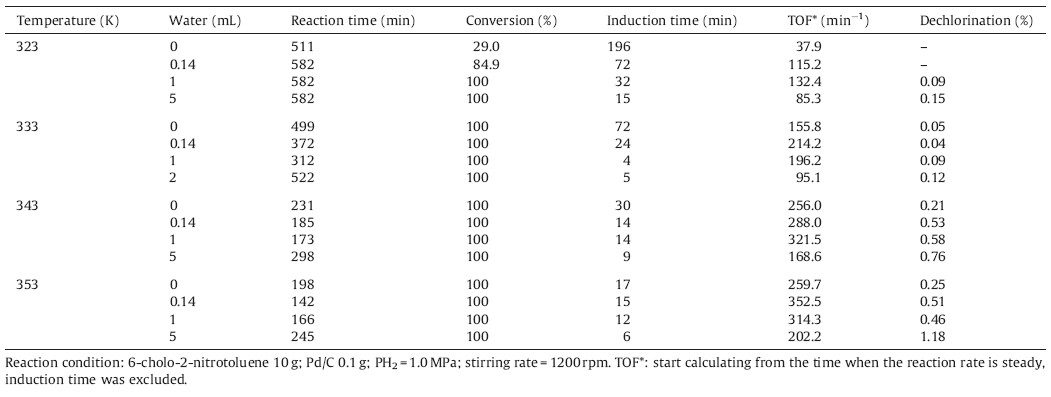
The density functional theory (DFT) calculations have been performed with the Vienna Ab Initio Simulation package (VASP) [21] and optB88-vdw exchange-correlation functions. A planewave basis set with a cutoff energy of 400 eV and ultrasoft Vanderbilt pseudopotentials (U.S.-PP) [23] was employed. A four layer 4 × 4 Pd (1 1 1) unit cell was used. The Brillouin zone integration was carried out with 4 × 4 × 1k-point sampling,where the error of total energy was smaller than 1.0 × 10-4 eV.
Dried sponge palladium (0.28 m2/g) was used as the catalyst for hydrogenation of 6-CNT under solvent-free conditions,because it is impossible to remove water (moisture) from Pd/C catalysts completely due to the hydrophilic covalently bound oxygen functionalities [22, 23],the porous structure and high surface area of activated carbon. It was found that no hydrogen was consumed and no product was detected after 480 min under 1.0 MPa H2 pressure at 353 K,indicating the hydrogenation of 6-CNT could not occur over dried sponge palladium. When minor amount of water (1 mL) was added,the hydrogenation of 6-CNT however could rapidly take place and the conversion of 6-CNT was 56.2% within only 130 min. The minor amount of water cannot be substituted by other solvents,for example cyclohexane. The addition of cyclohexane (5 mL) did not initiate the hydrogenation reaction without the addition of water and the conversion was lower when 1 mL of water was added. In general,the hydrogenation of halogenated nitrobenzenes observes Langmuir-Hinshelwood reaction mechanism [24]. The co-adsorption of halogenated nitrobenzenes and hydrogen on Pd catalysts is necessary for the reaction to occur. Therefore,density functional theory (DFT) calculations were carried out for the adsorption of halogenated nitrobenzenes (in the calculations,p-chloronitrobenzene was used),H2,H2O and the co-adsorption of p-chloronitrobenzene,H2 or H2O on Pd (111) surfaces. The adsorption energy of p-chloronitrobenzene on Pd (1 1 1) is -1.57 eV,which is much lower than that of hydrogen adsorption (-0.15 eV). The viscosity of halogenated nitrobenzenes (>3.3 mN S m-2 (298 K)) is much higher than that of the organic solvents like methanol (0.5 mN S m-2 (298 K)). It is anticipated that H2 will not be adsorbed on Pd (111) to an appreciable degree at the full coverage of halogenated nitrobenzenes. The adsorption strength of H2 decreases significantly (from -0.15 eV to -0.51 eV) in the presence of water and the adsorbed minor amount of water could allow the hydrogen to participate [25] in the hydrogenation reaction due to the dissociation of water,which initiates the reaction. In addition,H2 can diffuse and adsorb more easily on the Pd catalysts in the presence of water. Therefore,minor amount of water plays a significant role in the hydrogenation of halogenated nitrobenzenes under solvent-free conditions (Table 1).
| Table 1 The effect of minor water and/or cyclohexane on the hydrogenation of 6-CNT under solvent-free conditions over sponge palladium. |
Pd/C is more widely used as a catalyst than the sponge palladium,but moisture inevitably exists in the Pd/C catalysts. In order to reduce or eliminate the effect of inherent moisture in Pd/C, these catalysts were washed with cyclohexane five times before their use. The effect of the amount of feedstock and catalysts with a constant mass ratio (6-CNT: Pd/C = 100:1) on the reaction performance of the selective 6-CNT hydrogenation is shown in Table 2. When the amount of 6-CNT is 5 and 10 g,the induction time is nearly the same (about 70 min) and the average reaction rate does not change too much. When the amount of 6-CNT is 20 g or more,the induction time increases to more than 100 min. Here we chose 10 g as an optimal amount of feedstock,and the average reaction rate is measured to be 6.2 mol6-CNT/(molPd·min). The long induction time indicates the diffusion and adsorption of H2 is difficult under solvent-free conditions,as discussed in the above section (Fig. 1).
| Table 2 Effect of the different amounts of feedstock in Pd/C catalysts on the reaction process and catalytic performance of the 6-CNT selective hydrogenation. |
|
Download:
|
| Fig. 1.The optimized structures of p-chloronitrobenzene,co-adsorption p-chloronitrobenzene with hydrogen and water. | |
Fig. 2 shows the effect of different amounts of water on the hydrogen uptake of solvent-free 6-CNT hydrogenation at 323,333, 343 and 353 K,respectively. Firstly,the induction time dramatically decreases with the increase in temperature,which is 196,72, 30 and 17 min at 323,333,343 and 353 K,respectively (Fig. 2 and Table 3). The main reason is that at high temperatures,H2 can diffuse and adsorb on Pd catalysts more easily,which leads to shorter induction times. These results indicated the important role of H2O in the hydrogenation of halogenated nitrobenzenes. Secondly,at the same temperature,the induction time decreases with the increase in H2O amount. The promoting role of water is dependent on the temperature and water amount. At low temperatures (323,333 K) and small water amounts (0.14, 1 mL),the hydrogen uptake shows a non-linear tendency with respect to the reaction time. At high temperatures (343,353 K) or large amounts of water (5 mL),the hydrogen uptake shows nearly a linear tendency with respect to the reaction time,which indicates that the adsorbed and dissociated hydrogen was adequate for the reaction. Thirdly,irrespective of the temperature,the optimum amount of added water is determined to be between 0.14 mL and 1 mL based on the short induction reaction time and the high TOF. Meanwhile,the effect of minor amount of water on the selectivity of hydrogenation of 6-CNT is negligible for all the temperatures investigated. For Pd/C catalysts,the higher temperature and the larger amount of added water reduce the induction reaction time. When the amount of added water is between 0.14 mL and 1 mL, the highest TOF is obtained. All of the results indicate H2 diffusion in 6-CNT,adsorption and dissociation on Pd is easier at higher temperature with larger water amount. Therefore,the primary role of minor amount of water is to enhance the diffusion and adsorption of hydrogen on Pd/C catalysts [25] and allow hydrogen to more efficiently overcome the strong adsorption of halogenated nitrobenzenes [26].
| Table 3 Effects of the water amount on the hydrogenation process of 6-CNT at different temperature under solvent-free. |

|
Download:
|
| Fig. 2.Hydrogen uptake curve on the hydrogenation process of 6-CNT with different temperature and different water added under solvent-free. | |
In summary,our study shows water plays a very important role in solvent-free catalytic reactions,for example,the hydrogenation of halogenated nitrobenzenes. For dried sponge Pd,the reaction cannot occur without the addition of water. For Pd/C catalysts, minor amount of water increases the reaction rate and reaction TOFs and reduces the induction time.
This work was supported by the National Natural Science Foundation of China (Nos. 20976164,21176221 and 21136001), National Basic Research Program of China (973 Program) (Nos. 2011CB710803,2013CB733500) and Zhejiang Provincial Natural Science Foundation of China (No. LY12B03005).
| [1] | G.Y. Fan, L. Zhang, H.Y. Fu, et al., Hydrous zirconia supported iridium nanoparticles: an excellent catalyst for the hydrogenation of haloaromatic nitro compounds, Catal. Commun. 11 (2010) 451-455. |
| [2] | X.D. Wang, M.H. Liang, H.Q. Liu, Y. Wang, Selective hydrogenation of bromonitrobenzenes over Pt/gamma-Fe2O3, J. Mol. Catal. A-Chem. 273 (2007) 160-168. |
| [3] | C. Feng, H.Y. Zhang, N.Z. Shang, S.T. Gao, C. Wang, Magnetic graphene nanocomposite as an efficient catalyst for hydrogenation of nitroarenes, Chin. Chem. Lett. 24 (2013) 539-541. |
| [4] | X. Yuan, N. Yan, C.X. Xiao, et al., Highly selective hydrogenation of aromatic chloronitro compounds to aromatic chloroamines with ionic-liquid-like copolymer stabilized platinum nanocatalysts in ionic liquids, Green Chem. 12 (2010) 228-233. |
| [5] | M.H. Liang, X.D. Wang, H.Q. Liu, H.C. Liu, Y. Wang, Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes, J. Catal. 255 (2008) 335-342. |
| [6] | C.H. Liang, J.G. Han, K.H. Shen, et al., Palladium nanoparticle microemulsions: Formation and use in catalytic hydrogenation of o-chloronitrobenzene, Chem. Eng. J. 165 (2010) 709-713. |
| [7] | H.Q. Liu, M.H. Liang, C. Xiao, et al., An excellent Pd-based nanocomposite catalyst for the selective hydrogenation of para-chloronitrobenzene, J. Mol. Catal. A-Chem. 308 (2009) 79-86. |
| [8] | L.M. Sikhwivhilu, N.J. Coville, B.M. Pulimaddi, J. Venkatreddy, V. Vishwanathan, Selective hydrogenation of o-chloronitrobenzene over palladium supported nanotubular titanium dioxide derived catalysts, Catal. Commun. 8 (2007) 1999-2006. |
| [9] | X.C. Meng, H.Y. Cheng, S. Fujita, et al., Selective hydrogenation of chloronitrobenzene to chloroaniline in supercritical carbon dioxide over Ni/TiO2: significance of molecular interactions, J. Catal. 269 (2010) 131-139. |
| [10] | X.X. Han, H.R. Li, R.M. Zhou, Effect of rare earths on selective hydrogenation of pchloronitrobenzene over PtMOx/CNTs catalysts, Chin. Chem. Lett. 20 (2009) 96-98. |
| [11] | M. Sankar, N. Dimitratos, P.J. Miedziak, et al., Designing bimetallic catalysts for a green and sustainable future, Chem. Soc. Rev. 41 (2012) 8099-8139. |
| [12] | L. Kesavan, R. Tiruvalam, M.H. Ab Rahim, et al., Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles, Science 331 (2011) 195-199. |
| [13] | L. Ma, S. Chen, C.S. Lu, Q.F. Zhang, X.N. Li, Highly selective hydrogenation of 3,4- dichloronitrobenzene over Pd/C catalysts without inhibitors, Catal. Today 173 (2011) 62-67. |
| [14] | C.S. Lu, J.H. Lv, L. Ma, et al., Highly selective hydrogenation of halonitroaromatics to aromatic haloamines by ligand modified Ni-based catalysts, Chin. Chem. Lett. 23 (2012) 545-548. |
| [15] | C. Su, X.N. Li, Q.F. Zhang, et al., Behavior of adsorbed diphenyl-sulfide on the Pd/C catalyst for o-chloronitrobenzene hydrogenation, Chin. Chem. Lett. 24 (2013) 59- 62. |
| [16] | C. Lian, H.Q. Liu, C. Xiao, et al., Solvent-free selective hydrogenation of chloronitrobenzene to chloroaniline over a robust Pt/Fe3O4 catalyst, Chem. Commun. 48 (2012) 3124-3126. |
| [17] | M. Pietrowski, M. Zielinski, M. Wojciechowska, Selective reduction of chloronitrobenzene to chloroaniline on Ru/MgF2 catalysts, Catal. Lett. 128 (2009) 31-35. |
| [18] | J. Ning, J. Xu, J. Liu, et al., A remarkable promoting effect of water addition on selective hydrogenation of p-chloronitrobenzene in ethanol, Catal. Commun. 8 (2007) 1763-1766. |
| [19] | H.Y. Cheng, X.C. Meng, Y.C. Yu, F.Y. Zhao, The effect of water on the hydrogenation of o-chloronitrobenzene in ethanol, n-heptane and compressed carbon dioxide, Appl. Catal. A-Gen. 455 (2013) 8-15. |
| [20] | J.Y. Li, L. Ma, X.N. Li, C.S. Lu, H.Z. Liu, Effect of nitric acid, pretreatment on the properties of activated carbon and supported palladium catalysts, Ind. Eng. Chem. Res. 44 (2005) 5478-5482. |
| [21] | G. Kresse, J. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B 54 (1996) 11169-11186. |
| [22] | H.P. Boehm, Surface oxides on carbon and their analysis: a critical assessment, Carbon 40 (2002) 145-149. |
| [23] | R.H. Bradley, M.W. Smith, A. Andreu, M. Falco, Surface studies of novel hydrophobic active carbons, Appl. Surf. Sci. 257 (2011) 2912-2919. |
| [24] | B. Coq, F. Figueras, Structure-activity relationships in catalysis by metals: some aspects of particle size, bimetallic and supports effects, Coord. Chem. Rev. 178 (1998) 1753-1783. |
| [25] | L.R. Merte, G.W. Peng, R. Bechstein, et al., Water-mediated proton hopping on an iron oxide surface, Science 336 (2012) 889-893. |
| [26] | A. Gross, Ab initio molecular dynamics simulations of the adsorption of H-2 on palladium surfaces, ChemPhysChem 11 (2010) 1374-1381. |