Quaternary ammonium salts are compounds with the four hydrogen atoms of the ammonium ion substituted by hydrocarbon; generally similar with the nature of inorganic salts,easy to dissolve in the water,and the aqueous solution can be conductive [1]. As a quaternary ammonium salt,tetraethyl ammonium bromide is an important cationic surfactant,which is mainly used for emulsification,sterilization,etc. In recent years,it has also been applied in organic synthesis as a phase transfer catalyst,and has been becoming one of the important fine chemicals. The reported methods for the determination of quaternary ammonium salts mainly included spectrophotometry [2],high performance liquid chromatography [3],ion chromatography [4] and capillary electrophoresis [5]. The determinations of quaternary ammonium salts by spectrophotometry are generally complicated,while the determinations by chromatography are simple and have higher sensitivity. However,indirect ultraviolet detection requires added materials with ultraviolet absorption as background ultraviolet absorbing reagents in mobile phase to detect analytes not exhibiting ultraviolet absorption. The method is simple and fast,because the analyte derivatization process is not needed. At present,indirect ultraviolet detection is mainly combined with capillary electrophoresis [5, 6, 7],ion chromatography [8],ion-pair chromatography [9],high performance liquid chromatography [10],for the detection of inorganic ions and aliphatic hydrocarbons. The analysis of quaternary ammonium salt by ion-pair chromatography- indirect ultraviolet detection has scarcely been reported.
The goal of this work was to develop a method for the determination of tetraethyl ammonium (TEA),which has no ultraviolet absorption,by reversed-phase ion-pair chromatography with indirect ultraviolet detection. The separation and indirect ultraviolet detection of TEA were achieved on a reversed-phase C18 column using 4-aminophenol hydrochloride-1-heptanesulfonic acid sodium-methanol as the mobile phase at 230 nm wavelength. The method has been successfully applied to the analysis of tetraethyl ammonium bromide samples.
Methanol and acetonitrile (HPLC grade) were obtained from Dikma technologies (Shanghai,China). 1-Heptanesulfonic acid sodium and 1-pentanesulfonic acid sodium (HPLC grade) were obtained from Tianjin Guangfu Fine Chemical Research Institute (Tianjin,China). 4-Aminophenol hydrochloride,sulfosalicylic acid, nicotinamide,phthalic acid and acetic acid (analytical grade) were supplied by J&K Chemical,Ltd. (Beijing,China). Tetraethyl ammonium bromide (≥99%),used as the standard substance, was obtained from Shanghai Chengjie Chemical,Ltd. (Shanghai, China).
Standard solutions were prepared in 18.2 MΩ cm deionized distilled water from a Millipore Milli-Q water-purification system (Millipore,USA). Before injection,solutions were filtered through a 0.22 μm membrane filter (Shanghai Xinya Purity Instrument Factory,China). Stock standard solution of 1000 mg/L concentration was prepared. Working standard solution from stock solution was prepared on a daily basis as required.
Milli-Q water was also used to prepare mobile phases. Aqueous 4-aminophenol hydrochloride and 1-heptanesulfonic acid sodium solution were mixed in the appropriate concentration,and then acetic acid was added to adjust the required pH. A model PHSF-3F pH meter (Shanghai Precision and Scientific Instrument,Shanghai, China) was used for pH measurement. Before use,mobile phases were filtered through a 0.22 μm filter,and then degassed for 15 min with a Model DOA-P504-BN pump (IDEX,USA).
All experiments were carried out on an Agilent 1200 HPLC system (Agilent,USA),which consisted of a quaternary pump (Model Quat pump-G1311A),a detector (Model DAD-G1315D),an autosample injector (Model ALSG1329A),a column oven (Model TCC-G1316A) and a degasser system (Model Degasser-G1322A). The chromatographic system control,data acquisition and data analysis were performed using the Agilent Rev.B.04.01 workstation (Agilent,USA).
All separations were performed on a 4.6 mm i.d. × 150 mm ZORBAX Eclipse XDB C18 column (Agilent,USA). The optimal mobile phase consisted of 0.7 mmol/L 4-aminophenol hydrochloride and 0.15 mmol/L 1-heptanesulfonic acid sodium (pH 3.5) mixed with 20% (v/v) methanol. The flow rate was set at 1.0 mL/ min. Column temperature was 30℃. Injection volume was 20 mL. Ultraviolet detection (230 nm) was employed.
Because TEA has no ultraviolet absorption group in its molecular structure,the addition of background ultraviolet absorbing reagents to the mobile phase is needed for detection using the indirect ultraviolet detection method. The detection of TEA was investigated using 4-aminophenol hydrochloride,phthalic acid,nicotinamide and sulfosalicylic acid as background ultraviolet absorbing reagents at their respective maximum wavelengths. With the 0.5 mmol/L background ultraviolet absorbing reagent aqueous solution (adjusted to pH 3.0 with acetic acid) mixed with 20% methanol as the mobile phase,no chromatographic response for TEA was observed. Concerns that the appropriate ion-pair reagent in the mobile phase may act on the determination of TEA,further,0.5 mmol/L background ultraviolet absorbing reagent and 0.1 mmol/L 1-heptanesulfonic acid sodium (pH 3.0) mixed with 20% methanol was used as mobile phase. The results showed that only when the determination of TEA was performed using 4-aminophenol hydrochloride aqueous solution/1-heptanesulfonic acid sodium/methanol as mobile phase at 240 nm,we can clearly observe the chromatographic peak of TEA in the chromatogram. Therefore,in following investigations,we used 4-aminophenol hydrochloride as the background ultraviolet absorbing reagent.
In the investigation on detection wavelength,the flow rate was 1.0 mL/min,the column temperature was 30℃,and the mobile phase was 0.5 mmol/L 4-aminophenol hydrochloride and 0.1 mmol/L 1-heptanesulfonic acid sodium (pH 3.0) mixed with 20% methanol. It was found that using the detection wavelength at 230 nm,the baseline noise was the minimum with well-shaped peaks. Therefore,the detection wavelength of 230 nm was selected in the following investigations.
The effects of two ion-pair reagents 1-pentanesulfonic acid sodium and 1-heptanesulfonic acid sodium on the determination of TEA were also investigated.When using 1-pentanesulfonic acid sodium as the ion-pair reagent,the determination of TEA was not achieved. Therefore,1-heptanesulfonic acid sodium was chosen as ion-pairing reagent. In order to investigate the effect of 1- heptanesulfonic acid sodium concentrations on the determination of TEA and choose the optimal concentration,the concentrations of 1-heptanesulfonic acid sodium were varied from 0.05 mmol/L to 0.30 mmol/L using 1-heptanesulfonic acid sodium and 0.5 mmol/L 4-aminophenol hydrochloride (pH 3.0) mixed with 20% methanol as mobile phase. It was found that the retention time of TEA was lengthened with increasing concentrations of 1-heptanesulfonic acid sodium. As described in some reversed-phase ion-pair chromatography studies,the formation of neutral ion pairs is enhanced with increasing concentration of the ion-pair reagent (1-heptanesulfonic acid sodium),which strengthens the interaction between neutral ion-pair and stationary phase and thereby increases the retention of analyte (TEA). While the concentration was 0.05 mmol/L,the retention time of TEA was so short that the system peak interfered with the determination. When the concentration was 0.15 mmol/L,the baseline noise was the minimum with well-shaped peaks. Consequently,the appropriate concentration of 1-heptanesulfonic acid sodium was 0.15 mmol/L.
The effect of 4-aminophenol hydrochloride concentration on the determination of TEA was investigated using 4-aminophenol hydrochloride and 0.15 mmol/L 1-heptanesulfonic acid sodium (pH 3.0) mixed with 20% methanol as mobile phase. The concentrations of 4-aminophenol hydrochloride were investigated at 0.3,0.5,0.7 and 1.0 mmol/L. The results showed that the retention time of TEA was shortened with increasing the concentration of 4-aminophenol hydrochloride. Because the 4- aminophenol with positive charge in the mobile phase has the function of elution ion,so as its concentration increases,the elution ability of the mobile phase will also be enhanced. This phenomenon explains that the dynamic ion exchange mechanism exists in the process of TEA retention. At the concentration of 0.7 mmol/L, the baseline noise and the detection limit were the minimum. Therefore,the appropriate concentration of 4-aminophenol hydrochloride was 0.7 mmol/L.
The effect of the mobile phase pH on the determination of TEA was investigated using 0.7 mmol/L 4-aminophenol hydrochloride and 0.15 mmol/L 1-heptanesulfonic acid sodium mixed with 20% methanol as mobile phase. The result indicated that with the increase of aqueous solution pH of the mobile phase,the retention time will extend gradually from pH 3 to 4.5 and then reduce in pH 5. However,generally it will not significantly change,and when at pH 3.5,the baseline noise reaches the minimum level. Therefore,pH 3.5 was found to be suitable and so was used in further work.
Methanol and acetonitrile were used as organic solvents for the determination of TEA. When using acetonitrile as organic solvent, the peak of TEA was not observed. Therefore,methanol was selected as the organic solvent. In investigating the effects of methanol concentration,the volume fractions of methanol were 10%,20%,30%,35% and 40% with 0.7 mmol/L 4-aminophenol hydrochloride and 0.15 mmol/L 1-heptanesulfonic acid sodium (pH 3.5) as the mobile phase,the determination of TEA was performed. The results showed that the retention times of TEA were decreased with increasing methanol volume fractions. With increasing concentrations of methanol,the surface tension of the mobile phase is reduced,which in turn reduced the solvophobic adsorption of the neutral ion pair on the stationary phase,thus reduced retention. When the volume fractions of methanol were 35% and 40%,the TEA chromatographic peak partly overlapped with the system peak. When the volume fraction of methanol was 20%,the baseline noise and the detection limit were the minimum. Thus,the appropriate methanol concentration was 20%. In liquid chromatography,the relationship between retention factor (k) of analyte and the volume fraction of methanol φ (MeOH) in the mobile phase is lg k = cφ(MeOH) + d [11]. In this research,the equation is lg k = -3.6748φ(MeOH) + 0.9176 (r = 0.9977). According to the above data,the lg k versus φ (MeOH) showed better linearity,and the retention of TEA was comparable with the reversed-phase liquid chromatographic mode.
The effect of column temperature on the retention time of TEA was investigated with column temperatures set to 20,25,30,35 and 40℃. The mobile phase of 0.7 mmol/L 4-aminophenol hydrochloride and 0.15 mmol/L 1-heptanesulfonic acid sodium (pH 3.5) mixed with 20% methanol. As the results showed,the retention time of TEA decreased slightly from 3.043 min to 2.612 min with the increase of column temperature,but the effects of column temperature on the retention time of TEA were not obvious. Thus,further study confirmed 30℃ as optimal.
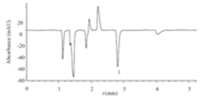
According to above the results and discussions,analysis conditions for TEA were well established by using 0.7 mmol/L 4- aminophenol hydrochloride and 0.15 mmol/L 1-heptanesulfonic acid sodium (pH 3.5) mixed with 20% methanol as mobile phase with the ultraviolet detection wavelength 230 nm and column temperature 30℃. Under the optimum conditions,the chromatogram of TEA standard solution is shown in Fig. 1. The retention time of TEA is 2.85 min. With quantitation by the use of peak area,the linear regression equation obtained from the relationship between the peak area (y,integral valve) and TEA concentration (x,mg/L) was y= 8.46x+ 1.98 with the correlation coefficient r of 0.9998 and the linear range of 0.20-120 mg/L. Calculations based on a S/N ratio of 3,the noise level was 0.0474 mAU,and the detection limit of TEA was 0.06 mg/L. This limit of detection is lower than conductivity detection [4]. The relative standard deviations (RSDs) of retention time and chromatographic peak area obtained by repeated measurement (n= 5) of TEA (27.7 mg/L) were 0.02% and 0.35%,respectively.
|
Download:
|
| Fig. 1.Chromatogram of a standard solution of tetraethyl ammonium. The chromatographic conditions: mobile phase,80% (0.7 mmol/L 4-aminophenol hydrochloride-0.15 mmol/L 1-heptanesulfonic acid sodium) aq. solution-20% (v/v) methanol; column,ZORBAX Eclipse XDB C18; flow rate,1.0 mL/min; column temperature,30℃; inject volume,20 μL; indirect ultraviolet detection,230 nm. Peak: 1,tetraethyl ammonium,TEA (27.7 mg/L). | |
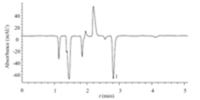
This method was applied to the determination of tetraethyl ammonium bromide synthesized by the chemistry lab. The tetraethyl ammonium bromide standard weight of 0.1003 g was diluted to 100 mL as stock solution. Then,a 2.5 aliquot of the stock solution was diluted to 50 mL and after filtering through a 0.22 μm membrane filter,the sample was used for the determination of TEA using the selected chromatographic conditions. The chromatogram is shown in Fig. 2. With quantitative use of the standard curve method,the content in the solution of sample was 27.7 mg/L.Recovery studies were tested by the standard addition method, and determined to be 99.1%. The above data were the average values of five measurements. The relative standard deviation was less than 1.0%. The results indicate this method has the advantages of high accuracy and good precision,and can meet the requirements for quantitative analysis of TEA.
|
Download:
|
| Fig. 2.Chromatogram of synthesized tetraethyl ammonium bromide sample.Chromatographic conditions and peak marks as in Fig. 1. | |
A method was developed for the determination of TEA by reversed-phase ion-pair chromatography with indirect ultraviolet detection using 4-aminophenol hydrochloride as the background ultraviolet absorbing reagent. Chromatographic separation was performed on a reversed-phase C18 column with 4-aminophenol hydrochloride-1-heptanesulfonic acid sodium-methanol as the mobile phase. The retention time of the TEA was 2.85 min,and the detection limit was 0.06 mg/L. The method has been successfully applied to determine the tetraethyl ammonium bromide synthesized by the chemistry lab. The accuracy and precision of the method can meet the requirements for the quantitative analysis of TEA.
This work was supported by the Natural Science Foundation of Heilongjiang Province (Grant No. B201307),the Ministry of Education of Heilongjiang Province (No. 12531192) and the Program for Scientific and Technological Innovation Team Construction in Universities of Heilongjiang Province (No. 2011TD010).
| [1] | T. Geng, X.G. Teng, Q.X. Li, Y.J. Jiang, G.J. Li, Synthesis and properties of long-chain quaternary ammonium hydroxides, Chin. Chem. Lett. 24 (2013) 494-496. |
| [2] | Y.P. Huang, N.X. Cai, A study on the determination of trace cetyltrimethylammonium on the basis of the discolor reaction of brilliant green, Anal. Sci. 17 (2001) 97-101. |
| [3] | Y.J. Dong, H. Yu, J.F. Wang, X. Huang, Analysis of tetramethyl ammonium ion by high performance liquid chromatography-indirect ultraviolet detection using ionic liquids as mobile phase, Chin. J. Anal. Chem. 39 (2011) 1251-1255. |
| [4] | L.L. Yang, X.Z. Zhang, H.F. Tian, Simultaneous determination of ammonium, tetraethylammonium and triethylmethylammonium by ion chromatography with non-suppressed conductivity detection, Chin, J. Inorg. Anal. Chem. 2 (2012) 43-46. |
| [5] | H.Y. Liu, W.H. Ding, Determination of homologues of quaternary ammonium surfactants by capillary electrophoresis using indirect UV detection, J. Chromatogr. A 1025 (2004) 303-312. |
| [6] | Z.X. Wang, Y. Chen, Q. Guo, Key conditions in capillary electrophoresis of amino acids with indirect ultraviolet detection, Chin. Chem. Lett. 11 (2000) 803-806. |
| [7] | M. Shi, Q.Y. Gao, J.M. Feng, Y.C. Lu, Analysis of inorganic cations in honey by capillary zone electrophoresis with indirect UV detection, J. Chromatogr. Sci. 50 (2012) 547-552. |
| [8] | C. Fernandes, R.S. Leite, F.M. Lanças, Rapid determination of bisphosphonates by ion chromatography with indirect UV detection, J. Chromatogr. Sci. 45 (2007) 236-241. |
| [9] | X. Huang, H. Yu, Y.J. Dong, Rapid and simultaneous determination of imidazolium and pyridinium ionic liquid cations by ion-pair chromatography using a monolithic column, Chin. Chem. Lett. 23 (2012) 843-846. |
| [10] | L.N. Li, Z.K. Duan, H.Y. Zeng, Y.P. He, Q.Y. Chen, Determination of hexane in the acid cluster of caprolactam by high performance liquid chromatography with indirect photometric detection, Chin. J. Chromatogr. 29 (2011) 87-90. |
| [11] | M.J. Ruiz-Angel, A. Berthod, Reversed-phase liquid chromatography alkyl-imidazolium ionic liquids, J. Chromatogr. A 1113 (2006) 101-108. |




