b State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, China
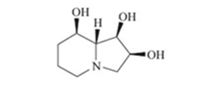
(-)-Swainsonine (Fig. 1) is an indolizidine alkaloid that is also classified as an azasugar (imino sugar) [1]due to the presence of three hydroxyl groups in the molecule. After its first isolation in 1973 from the fungus Rhizoctomia leguminicola [2a],it has also been extracted from diverse fungi and numerous species of flowering plants [2b,1a]. As an azasugar,(-)-swainsonine exhibits lysosomal a-mannosidase and mannosidase II inhibitory properties. Although the pharmacological properties of this product have not been fully investigated,it has been tested as a treatment for cancer [3],HIV,and immunological disorders [1,4a]. The important biological properties of swainsonine have attracted the interest of many synthetic and medicinal chemists. Numerous methods have been developed for the stereoselective synthesis of swainsonine and its diastereomers [4, 5, 6, 7]. In connection with a general program on the development of efficient and general methodologies for the synthesis of N-containing bioactive compounds and alkaloids [8], we became interested in the stereoselective synthesis of (-)- swainsonine,and have recently reported the synthesis of two diastereomers of (-)-swainsonine [9]. We now report a short formal stereoselective synthesis of (-)-swainsonine.
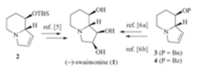
A survey of literature revealed that among the many approaches to swainsonine [4, 5, 6, 7],the unsaturated indolizidine derivatives 2 [5],3 [6a],and 4 [6b]proved to be reliable advanced intermediates for the synthesis of swainsonine (Scheme 1). Since indolizidine 2 is a silica gel sensitive compound [5d],we chose the unsaturated indolizidine 3 as our target in view of developing a short formal stereoselective synthesis of (-)-swainsonine.
|
Download:
|
| Scheme 1.Typical synthetic approaches to (-)-swainsonine based on the unsaturated indolizidines 2-4. | |
To a solution of anhydrous zinc chloride (1.0 mol/L in diethyl ether,3.6 mL. 3.6 μmol) in dichloromethane (0.5 mL) was added dropwise an Et2O solution of vinylmagnesium bromide (1.0 mol/L in diethyl ether,6.0 mL,6.0 μmol). The mixture was stirred at room temperature under nitrogen for 30 min. A solution of a diastereomeric mixture of sulfone 8 (1.16 g,3.01 μmol) in anhydrous dichloromethane (8 mL) was added and the mixture was stirred at room temperature for 14-16 h. The reaction was quenched with a saturated aqueous NH4Cl and the resulting mixture was extracted with dichloromethane (3×25 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel (eluent: EtOAc/ PE = 1/4) to give an inseparable diastereomeric mixture of trans-7 and cis-7 as a colorless oil (612 mg,combined yield: 75%,trans/ cis = 6/1). IR (film,cm-1): νmax 2925,1723,1652,1457,1403,1358, 1266,1076,922,730,698; 1HNMR(400 MHz,CDCl3): δ(data of the major diastereomer read from the spectrum of the diastereomeric mixture) 1.96 (dt,2H,J = 8.8,4.4 Hz),2.35 (dt,1H,J = 18.0,4.4 Hz), 2.65 (dt,1H,J = 18.0,9.6 Hz),3.18 (dd,1H,J = 15.6,7.2 Hz),3.65 (dd,1H,J = 5.6,2.8 Hz),4.11 (d,1H,J = 5.6 Hz),4.56 (d,1H, J = 12.8 Hz),4.60 (d,1H,J = 12.8 Hz),4.77 (dt,1H,J = 15.6,2.0 Hz), 5.11-5.29 (m,4H),5.61-5.79 (m,1H),7.26-7.35 (m,5H); 13C NMR (100 MHz,CDCl3): δ 21.4,27.1,47.1,62.2,70.3,73.9,116.9,118.2, 127.4 (2C),127.7,128.4 (2C),132.9,135.8,138.0,169.6; HRESIMS calcd. for [C17H21NNaO2]+ (M+Na+): 294.1465; found: 294.1470.
A solution of a diastereomeric mixture of 6-vinylpiperidin-2- one 7 (116.7 mg,0.43 μmol) in degassed CH2Cl2 (8 mL) containing Grubbs second generation catalyst 10 (36 mg,0.043 μmol) was stirred for 12 h at refluxing. The solution was concentrated and the resulting residue was purified by flash chromatography on silica gel (eluent: EtOAc/PE = 1/3) to give trans-6 (83 mg,yield: 80%) and cis-6 (14 mg,yield: 13%).
trans-6: colorless oil. [α]D20 -110.1 (c 0.33,CHCl3); IR (film, cm-1): νmax 2925,2847,1648,1611,1441,1407,1096,1063,740, 698; 1H NMR (400 MHz,CDCl3): d 1.78-1.88 (m,1H),2.17-2.23 (m, 1H),2.40 (dt,1H,J = 17.6,8.0 Hz),2.62 (ddd,1H,17.6,8.0,4.8 Hz), 3.41 (ddd,1H,J = 14.4,9.2,5.6 Hz),4.04 (d,1H,J = 16.0 Hz),4.27- 4.28 (m,1H),4.44 (dt,1H,J = 16.0,2.2 Hz),4.52 (d,1H,J = 11.6 Hz), 4.68 (d,1H,J = 11.6 Hz),5.88-5.93 (m,1 H),6.01-6.05 (m,1H), 7.28-7.38 (m,5H); 13C NMR (100 MHz,CDCl3): d 26.4,29.7,52.9, 67.4,71.3,77.1,126.9,127.7 (2C),127.9,128.3,128.5 (2C),137.9, 168.7; HRESIMS calcd. for [C15H17NNaO2]+ (M+Na+): 266.11515; found: 266.11514.
cis-6: colorless oil. [α]D20 -8.5 (c 0.8,CHCl3) {[α]D20 -8.4 (c 1.31, CHCl3) [14]}; 1HNMR(400 MHz,CDCl3): d 1.77-1.98 (m,1H),2.09- 2.26 (m,1H),2.44-2.57 (m,2H),3.93-3.98 (m,1H),4.05 (d,1H, J = 16.0 Hz),4.39-4.45 (m,1H),4.49 (d,1H,J = 12.4 Hz),4.59 (dt, 1H,J = 16.0,2.4 Hz),4.60 (d,1H,J = 12.4 Hz),5.76-5.81 (m,1H), 5.93-5.98 (m,1H),7.25-7.36 (m,5H); 13C NMR (100 MHz,CDCl3): d 24.8,27.0,53.0,68.0,70.5,70.7,127.0,127.3,127.4 (2C),127.7, 128.4 (2C),138.3,169.1; HRESIMS calcd. for [C15H17NNaO2]+ (M+Na+): 266.11515; found: 266.11515.
|
Download:
|
| Fig. 1.The structure of (-)-swainsonine (1). | |
To an ice-cooled,stirred solution of indolizidinone trans-6 (25.9 mg,0.11 μmol) in THF (2 mL) was added LiAlH4 (20.0 mg, 0.53 μmol),and the mixture was stirred at room temperature for 4 h. The reaction was quenched with a saturated aqueous NaHCO3 at 0℃. The resulting slurry was filtered through a celite pad and washed with EtOAc (5 mL). The filtrate was extracted with EtOAc (3× 5 mL),and the combined organic extracts were dried over anhydrous Na2SO4,filtered and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel (EtOAc/PE = 1/1) to give compound 3 (22 mg,yield: 89%) as a colorless oil: [α]D20 -115 (c 1.0,CHCl3) {[α]D20 -115 (c 3.85, CHCl3) [6a]}; IR (film,cm-1): νmax 3058,3029,2925,2851,2772, 2751,1635,1494,1449,1192,1088,889,731,694; 1H NMR (400 MHz,CDCl3): δ 1.14-1.32 (m,1H),1.52-1.74 (m,2H),2.20 (ddd,1H,J = 11.7,7.1,3.9 Hz),2.43 (dt,1H,J = 11.4,3.2 Hz),2.94 (dd,1H,J = 11.4,3.6 Hz),2.97-3.04 (m,1H),3.23-3.32 (m,2H),3.63 (d,1H,J = 13.2 Hz),4.54 (d,1H,J = 12.0 Hz),4.66 (d,1H,J = 12.0 Hz), 5.89 (ddd,1H,J = 6.0,4.0,2.0 Hz),6.14 (dd,1H,J = 6.0,0.8 Hz), 7.20-7.36 (m,5H); 13C NMR (100 MHz,CDCl3): δ 24.2,30.4,48.9, 57.7,71.0,72.1,78.5,127.5,127.6 (2C),128.4 (2C),128.8,131.4, 138.9; HRESIMS calcd. for [C15H20NO]+ (M+H+): 230.1539; found:230.1540.
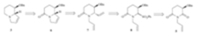
Our retrosynthetic analysis of indolizidine 3 is outlined in Scheme 2. The essential of this analysis resides on the use of (R)- benzyloxyglutarimide (5),a versatile chiral building block developed from our laboratory as a source of chirality for (-)- swainsonine [10]. Indolizidine 3 can be derived from indolizidinone 6. The pyrroline moiety in indolizidinone 6 is accessible by the RCM reaction from diene 7 [11],and one vinyl group in 7 can be introduced by the Ley’s sulfone-based chemistry [12].
|
Download:
|
| Scheme 2.Retrosynthetic analysis of indolizidine 3. | |
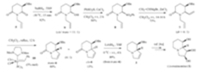
The synthesis commenced with the regio- and diastereoselective reduction [10a]of the known chiral building block (R)- benzyloxyglutarimide 5 [10b](NaBH4,THF,-30℃,10 min),which produced the hemiaminal 9 as a diastereomeric mixture (dr = 11:1) in a combined yield of 82% (Scheme 3). The major diastereomer was tentatively assigned as cis in light of our previous results on a similar system [10b]. Without separation,the diastereomeric mixture [13, 14]of 9 was treated with phenylsulfinic acid and CaCl2 [12a]in CH2Cl2 at r.t. for 2 h to give the sulfone 8 in a yield of 86%. Although sulfone 8 was obtained as an inseparable diastereomeric mixture,the diastereomeric mixture can be used in the next step without separation. The subsequent reaction is considered to pass through an N-acyliminium intermediate [10, 13],either diastereomer could give the same N-acyliminium ion. On standing at -20℃ for two weeks,the minor diastereomer in the diastereomeric mixture was epimerized gradually and completely to give the trans-diastereomer. This is in accordance with the phenomenon we observed previously on the corresponding 5-phenylsulfonyl-pyrrolidin-2-one homologue [12b]. Reaction of the diastereomeric mixture of 6-phenylsulfonyllactam 8 with organozinc reagent,generated in situ from vinylmagnesium bromide and a 1.0 mol/L solution of anhydrous ZnCl2 in diethyl ether [12b],at r.t. for 14-16 h yielded 6- vinyllactam 7 in 75% yield as an inseparable 6:1 diastereomeric mixture (determined by 1H NMR). The stereochemistry of the major diastereomer was tentatively deduced as trans based on our previous results with the pyrrolidinone homologous [12b,12d], which was confirmed by converting the diastereomeric mixture 7 into the known compounds cis-6 [14]and 3 [6a],respectively.
|
Download:
|
| Scheme 3.Formal stereoselective synthesis of (-)-swainsonine (1). | |
We next investigated the RCM reaction [8b,11]. Treatment of the diastereomeric mixture of diene 7 with Grubbs second generation catalyst [15]10 in CH2Cl2 at reflux produced the desired unsaturated indolizidinones trans-6 and cis-6 (ratio = 6:1) in a combined yield of 93%. The physical and spectral data of cis-6 match those reported {[α]D20 -8.5 (c 0.8,CHCl3); [α]D20 -8.4 (c 1.31, CHCl3) [15]}. Reduction of indolizidinone trans-6 with LiAlH4 in THF provided the known unsaturated (8R,8aS)-indolizidine (3) in an 89% yield. The physical and spectral (1H NMR and 13C NMR) data of the synthetic indolizidine 3 are in agreement with those reported {[α]D20 -115 (c 1.0,CHCl3); [α]D20 -115 (c 3.85,CHCl3)} [6a]. Thus,the stereochemistry assigned for trans-6 and cis-6 was further confirmed. Since the unsaturated indolizidine (8R,8aS)-3 has been converted by Pyne and co-workers in four steps into (-)- swainsonine (1) [6a],our synthesis thus constitutes a short formal stereoselective synthesis of this alkaloid.
In summary,we have developed a five-step synthesis of the unsaturated indolizidine (8R,8aS)-3,and thus accomplished a short formal stereoselective synthesis of (-)-swainsonine (1). Through this work,we have demonstrated that a combination of the versatile building block (R)-5 with the Ley’s-sulfone chemistry and the RCM reaction constitutes a powerful method for a rapid access to the highly functionalized 8-oxygenated indolizidin-5-one 6, which may be used as a versatile intermediate for the stereoselective synthesis of other hydroxylated indolizidine alkaloids.
| [1] | (a) B.G. Winchester, Iminosugars: from botanical curiosities to licensed drugs, Tetrahedron: Asymmetry 20 (2009) 645-651; (b) H. Chen, D.Y. Wang, X.L. Li, Advances in glycosyltransferase inhibitors, Chin. J. Org. Chem. 29 (2009) 703-715. |
| [2] | (a) F.P. Guengerich, S.J. DiMari, H.P. Broquist, Isolation and characterization of a lpyrindine fungal alkaloid, J. Am. Chem. Soc. 95 (1973) 2055-2056; (b) S.M. Colegate, P.R. Dorling, C.R. Huxtable, A spectroscopic investigation of swainsonine: an a-mannosidase inhibitor isolated from Swainsona canescens, Aust. J. Chem. 32 (1979) 2257-2264. |
| [3] | (a) P.R. Dorling, C.R. Huxtable, P. Vogel, Lysosomal storage in Swainsona spp. toxicosis: an induced mannosidosis, Neuropathol. Appl. Neurobiol. 4 (1978) 285- 295; (b) P.E. Shaheen, W. Stadler, P. Elson, et al., Phase Ⅱ study of the efficacy and safety of oral GD0039 in patients with locally advanced or metastatic renal cell carcinoma, Invest. New Drugs 23 (2005) 577-581. |
| [4] | (a) A.E. Nemr, Synthetic methods for the stereoisomers of swainsonine and its analogues, Tetrahedron 56 (2000) 8579-8629; (b) S.G. Pyne, Recent developments on the synthesis of (—)-swainsonine and analogues, Curr. Org. Synth. 2 (2005) 39-57; (c) L.F. Nie, H. Ba, A. Haji, Progress in total synthesis of swainsonine, Chin. J. Org. Chem. 29 (2009) 1354-1361. |
| [5] | (a) G. Archibald, C.P. Lin, P. Boyd, D. Barker, V. Caprio, A divergent approach to 3- piperidinols: a concise syntheses of (+)-swainsonine and access to the 1-substituted quinolizidine skeleton, J. Org. Chem. 77 (2012) 7968-7980; (b) H.G. Choi, J.H. Kwon, J.C. Kim, et al., A formal synthesis of (Ⅲ-swainsonine from a chiral aziridine, Tetrahedron Lett. 51 (2010) 3284-3285; (c) R.W. Bates, M.R. Dewey, A formal synthesis of swainsonine by gold-catalyzed allene cyclization, Org. Lett. 11 (2009) 3706-3708; (d) H.Y. Kwon, C.M. Park, S.B. Lee, J.H. Youn, S.H. Kang, Asymmetric iodocyclization catalyzed by salen-CrⅢCl: its synthetic application to swainsonine, Chem. Eur. J. 14 (2008) 1023-1028; (e) C.W.G. Au, S.G. Pyne, Asymmetric synthesis of anti-1 2-amino alcohols via the borono-Mannich reaction: a formal synthesis of (Ⅲ-swainsonine, J. Org. Chem. 71 (2006) 7097-7099; (f) J. Seayad, B. List, Asymmetric organocatalysis, Org. Biomol. Chem. 3 (2005) 719-724; (g) N. Buschmann, A. Rückert, S. Blechert, A new approach to (Ⅲ-swainsonine by ruthenium-catalyzed ring rearrangement, J. Org. Chem. 67 (2002) 4325-4329; (h) I. Dechamps, O.G. Pardo, J. Cossy, Enantioselective ring expansion of prolinol derivatives. Two formal syntheses of (Ⅲ-swainsonine, Tetrahedron 63 (2007) 9082-9091; (i) J. de Vicente, R.G. Arrayás, J. Cañada, J.C. Carretero, Stereoselective synthesis of (Ⅲ-swainsonine and 1,2-di-epi-swainsonine from g-hydroxy-a,b-unsaturated sulfones, Synlett (2000) 53-56; (j) T. Oishi, T. Iwakuma, M. Hirama, S. Itô, Stereoselective synthesis of (+)- swainsonine and (Ⅲ-8,8a-di-epi-swainsonine, Synlett (1995) 404-406. |
| [6] | (a) K.B. Lindsay, S.J. Pyne, Asymmetric synthesis of (Ⅲ-swainsonine, (+)-1,2-diepi- swainsonine, and (+)-1,2,8-tri-epi-swainsonine, J. Org. Chem. 67 (2002) 7774-7780; (b) M.J. Chen, Y.M. Tsai, Radical cyclizations of acylsilanes in the synthesis of (+)- swainsonine and formal synthesis of (Ⅲ-epiquinamide, Tetrahedron 67 (2011) 1564-1574. |
| [7] | (a) V. Dhand, J.A. Draper, J. Moore, R. Britton, A short, organocatalytic formal synthesis of (Ⅲ-swainsonineand related alkaloids,,Org. Lett.15(2013) 1914-1917; (b) J. Louvel, F. Chemla, E. Demont, F. Ferreira, A. Pérez-Luna, Synthesis of (Ⅲ- swainsonine and (Ⅲ-8-epi-swainsonine by the addition of allenylmetals to chiral a,b-alkoxy sulfinylimines, Org. Lett. 13 (2011) 6452-6455; (c) D.J. Wardrop, E.J. Bowen, Nitrenium ion-mediated alkene bis-cyclofunctionalization: total synthesis of (Ⅲ-swainsonine, Org. Lett. 13 (2011) 2376- 2379; (d) X.L. Wang, W.F. Huang, X.S. Lei, et al., A facile synthesis of 1,4-dideoxy- 1,4-imino-L-ribitol (LRB) and (Ⅲ-8a-epi-swainsonine from D-glutamic acid, Tetrahedron 67 (2011) 4919-4923; (e) S.J. Oxenford, S.P. Moore, G. Carbone, et al., Asymmetric synthesis via aziridinium ions: exploring the stereospecificity of the ring opening of aziridinium ions and a formal synthesis of (Ⅲ-swainsonine, Tetrahedron: Asymmetry 21 (2010) 1563-1568; (f) Y.S. Tian, J.E. Joo, B.S. Koog, et al., Asymmetric synthesis of (Ⅲ-swainsonine, J. Org. Chem. 74 (2009) 3962-3965; (g) H.B. Guo, G.A. O'Doherty, De novo asymmetric syntheses of D-, L- and 8- epi-D-swainsonine, Tetrahedron 64 (2008) 304-313. |
| [8] | (a) K.J. Xiao, Y. Wang, Y.H. Huang, X.G. Wang, P.Q. Huang, A direct and general method for the reductive alkylation of tertiary lactams/amides: application to the step economical synthesis of alkaloid (Ⅲ-morusimic acid D, J. Org. Chem. 78 (2013) 8305-8311; (b) K.J. Xiao, J.M. Luo, X.E. Xia, Y. Wang, P.Q. Huang, General one-pot reductive gem-bis-alkylation of tertiary lactams/amides: rapid construction of 1-azaspirocycles and formal total synthesis of (±)-cephalotaxine, Chem. Eur. J. 19 (2013) 13075-13086; (c) X.J. Dai, P.Q. Huang, A short and flexible synthetic approach to the naturally occurring racemic neoclausenamide and its analogs, Chin. J. Chem. 30 (2012) 1953-1956; (d) J. Chen, A.E. Wang, H.H. Huo, P.Q. Huang, Progress on the total synthesis of natural products in China: from 2006 to 2010, Sci. China-Chem. 55 (2012) 1175- 1212; (e) Y.H. Wang, W. Ou, L.F. Xie, J.L. Ye, P.Q. Huang, Towards reaction control: cisdiastereoselective reductive dehydroxylation of 5-alkyl-4-benzyloxy-5-hydroxy-2- pyrrolidinones, Asian J. Org. Chem. 1 (2012) 359-365; (f) K.J. Xiao, Y.H. Huang, P.Q. Huang, General direct transformation of secondary amides to ketones via amide activation, Acta Chim. Sin. 70 (2012) 1917-1922; (g) B. Teng, J.F. Zheng, H.Y. Huang, P.Q. Huang, Enantioselective synthesis of glutarimide alkaloids cordiarimides A, B, crotonimides A, B, and julocrotine, Chin. J. Chem. 29 (2011) 1312-1318; (h) Q.L.Peng,S.P.Luo,X.E.Xia,L.X.Liu,P.Q.Huang,Thefour-steptotalsynthesisof (-)- chaetominine, Chem. Commun. 49 (2013), http://dx.doi.org/10.1039/C3CC48833K. |
| [9] | H.K. Zhang, S.Q. Xu, J.J. Zhuang, J.L. Ye, P.Q. Huang, A flexible enantioselective approach to 3,4-dihydroxyprolinol derivatives by SmI2-mediated reductive coupling of chiral nitrone with ketones/aldehydes, Tetrahedron 68 (2012) 6656- 6664. |
| [10] | (a) P.Q. Huang, Asymmetric synthesis of hydroxylated pyrrolidines, piperidines and related bioactive compounds: from N-acyliminium chemistry to N-伪-carbanion chemistry, Synlett (2006) 1133-1149; (b) H.K. Zhang, X. Li, H. Huang, P.Q. Huang, Asymmetric syntheses of (8R,8aS)- and (8R,8aR)-8-hydroxy-5-indolizidinones: two promising oxygenated indolizidine building blocks, Sci. Sin. Chim. 41 (2011) 732-740 (in Chinese); (c) H.K. Zhang, X. Li, H. Huang, P.Q. Huang, Asymmetric syntheses of (8R,8aS)- and (8R,8aR)-8-hydroxy-5-indolizidinones: two promising oxygenated indolizidine building blocks, Sci. China-Chem. 54 (2011) 737-744. |
| [11] | (a) A. Deiters, S.F. Martin, Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis, Chem. Rev. 104 (2004) 2199-2238; (b) F.X. Felpin, J. Lebreton, Recent advances in the total synthesis of piperidine and pyrrolidine natural alkaloids with ring-closing metathesis as a key step, Eur. J. Org. Chem. (2003) 3693-3712. |
| [12] | (a) D.S. Brown, P. Charreau, T. Hansson, S.V. Ley, Substitution reactions of 2- phenylsulphonyl-piperidines and -pyrrolidines with carbon nucleophiles: synthesis of the pyrrolidine alkaloids norruspoline and ruspolinone, Tetrahedron 47 (1991) 1311-1328; (b) P.Q. Huang, X. Tang, A.Q. Chen, An alternative stereoselective synthesis of protected trans-5-alkyl-4-hydroxy-2-pyrrolidinones, Synth. Commun. 13 (2000) 2259-2268; (c) G.W. David, W.H. Richard Jr., S.F. Martin, Concise formal synthesis of (Ⅲ- peduncularine via ring-closing metathesis, Org. Lett. 5 (2003) 3523-3525; (d) X.G. Wang, A. E Wang, Y. Hao, Y.P. Ruan, P.Q. Huang, Modular enantioselective synthesis of 8-aza-prostaglandin E1, J. Org. Chem. 78 (2013) 9488-9493. |
| [13] | (a) A. Yazici, S.G. Pyne, Intermolecular addition reactions of N-acyliminium ions (part I), Synthesis (2009) 339-368; (b) A. Yazici, S.G. Pyne, Intermolecular addition reactions of N-acyliminium ions (part Ⅱ, Synthesis (2009) 513-541. |
| [14] | G.C. Fu, S.T. Nguyen, R.H. Grubbs, Catalytic ring-closing metathesis of functionalized dienes by a ruthenium carbene complex, J. Am. Chem. Soc. 115 (1993) 9856-9857. |
| [15] | H.K. Lee, J.S. Chun, C.S. Pak, Facile transformation of 2-azetidinones to 2-piperidones: application to the synthesis of the indolizidine skeleton and (8S,8aS)- perhydro-8-indolizinol, J. Org. Chem. 68 (2003) 2471-2474. |




